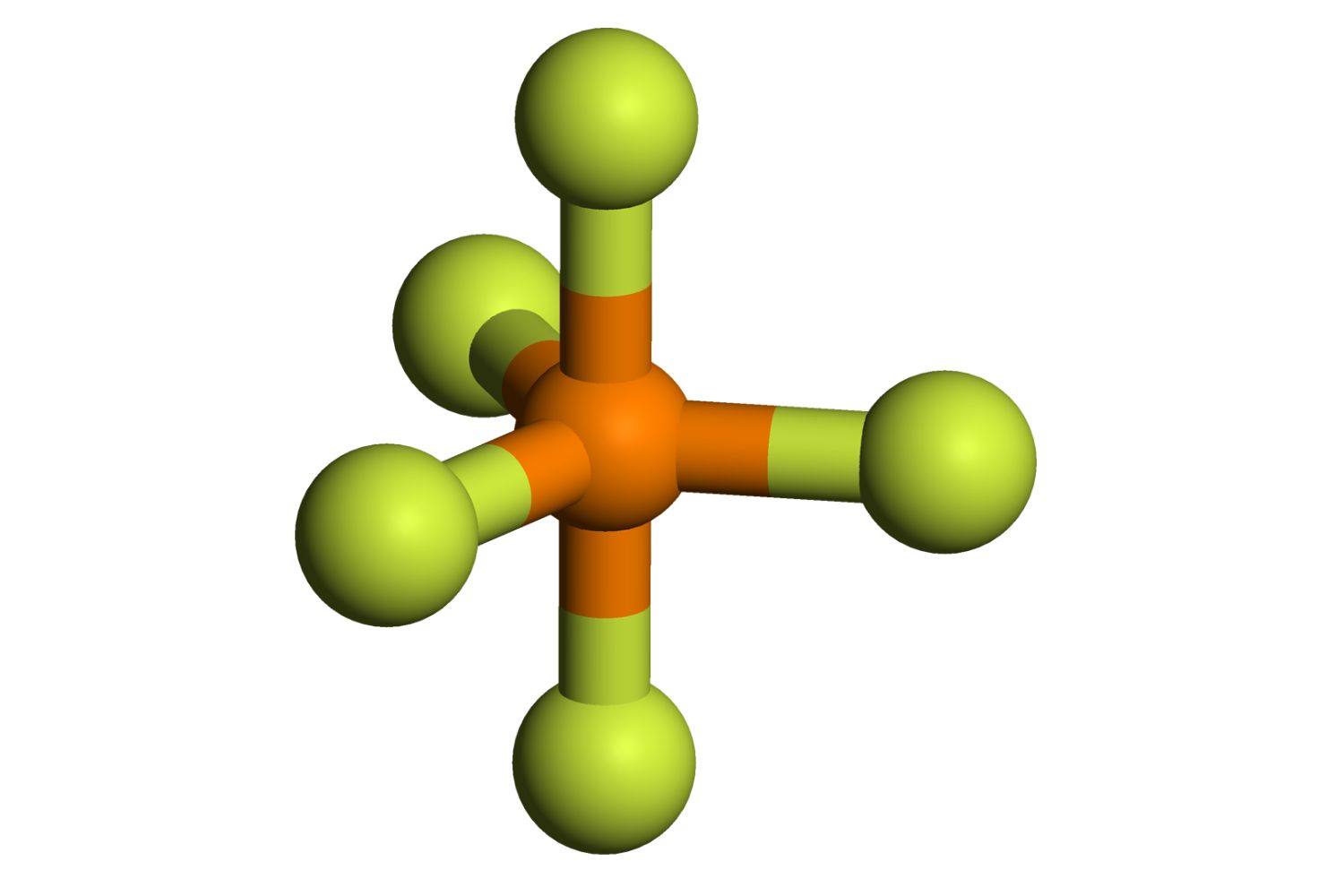
Phosphorus Pentafluoride might sound like a mouthful, but it's a fascinating compound with some surprising properties. What is Phosphorus Pentafluoride? Phosphorus Pentafluoride (PF5) is a colorless gas used in various chemical reactions and industries. This compound consists of one phosphorus atom surrounded by five fluorine atoms, forming a trigonal bipyramidal shape. It's known for being highly reactive and can act as a powerful fluorinating agent. Despite its usefulness, PF5 must be handled with care due to its toxicity and potential to cause respiratory issues. Ready to learn more? Let's dive into 40 intriguing facts about this unique chemical!
Key Takeaways:
- Phosphorus Pentafluoride (PF5) is a highly reactive compound with a pungent odor, used in catalysis, semiconductor industry, and chemical research. It requires careful handling due to its toxicity and corrosiveness.
- PF5 has a trigonal bipyramidal structure, serves as a Lewis acid, and is involved in gas-phase chemistry experiments. It's a valuable tool in studying fluorine chemistry, but its hazardous nature requires strict regulations and proper safety measures.
What is Phosphorus Pentafluoride?
Phosphorus pentafluoride (PF5) is a chemical compound with intriguing properties and uses. Let's dive into some fascinating facts about this compound.
-
Chemical Formula: The chemical formula for phosphorus pentafluoride is PF5.
-
Molecular Weight: PF5 has a molecular weight of approximately 125.97 g/mol.
-
Appearance: This compound is a colorless gas at room temperature.
-
Odor: PF5 has a pungent odor, similar to that of other phosphorus compounds.
-
Boiling Point: The boiling point of PF5 is -84.5°C (-120.1°F).
-
Melting Point: It has a melting point of -93.78°C (-136.8°F).
-
Density: The density of PF5 gas is about 5.527 g/L at standard temperature and pressure.
-
Solubility: PF5 is soluble in organic solvents like benzene and carbon tetrachloride.
Chemical Properties of Phosphorus Pentafluoride
Understanding the chemical properties of PF5 helps in grasping its reactivity and applications.
-
Reactivity: PF5 is highly reactive and can form complexes with many other compounds.
-
Hydrolysis: It hydrolyzes rapidly in water to form phosphoric acid and hydrofluoric acid.
-
Lewis Acid: PF5 acts as a strong Lewis acid, meaning it can accept electron pairs.
-
Oxidation State: In PF5, phosphorus is in the +5 oxidation state.
-
Bonding: The molecule has a trigonal bipyramidal structure, with phosphorus at the center.
-
Electronegativity: The electronegativity of phosphorus in PF5 is 2.19.
-
Fluorine Bonds: Each phosphorus atom in PF5 is bonded to five fluorine atoms.
Uses of Phosphorus Pentafluoride
PF5 has several practical applications in various fields due to its unique properties.
-
Catalysis: It is used as a catalyst in organic synthesis reactions.
-
Fluorinating Agent: PF5 serves as a fluorinating agent in chemical reactions.
-
Semiconductor Industry: It is used in the semiconductor industry for etching processes.
-
Chemical Research: Researchers use PF5 to study reaction mechanisms and molecular structures.
-
Gas-Phase Chemistry: PF5 is involved in gas-phase chemistry experiments.
-
NMR Spectroscopy: It is used in nuclear magnetic resonance (NMR) spectroscopy as a reference compound.
-
Pharmaceuticals: PF5 is sometimes used in the synthesis of pharmaceutical intermediates.
Safety and Handling of Phosphorus Pentafluoride
Handling PF5 requires caution due to its reactivity and potential hazards.
-
Toxicity: PF5 is highly toxic and can cause severe respiratory issues if inhaled.
-
Corrosiveness: It is corrosive to metals and tissues, requiring proper protective equipment.
-
Storage: PF5 should be stored in a cool, dry place away from moisture.
-
Handling: Use in a well-ventilated area with appropriate safety measures.
-
First Aid: In case of exposure, seek immediate medical attention and follow safety protocols.
-
Environmental Impact: PF5 can have harmful effects on the environment if not handled properly.
Interesting Facts about Phosphorus Pentafluoride
Here are some lesser-known yet intriguing facts about PF5.
-
Discovery: PF5 was first synthesized in the early 20th century.
-
Isotopes: Phosphorus in PF5 can exist in different isotopic forms, affecting its properties.
-
Spectral Properties: PF5 has distinct spectral properties used in various analytical techniques.
-
Molecular Geometry: The trigonal bipyramidal geometry of PF5 is a classic example in chemistry education.
-
Bond Angles: The bond angles in PF5 are 90° and 120°, depending on the position of the fluorine atoms.
-
VSEPR Theory: PF5 is often used to explain the Valence Shell Electron Pair Repulsion (VSEPR) theory.
-
Industrial Production: PF5 is produced industrially by reacting phosphorus pentachloride with hydrogen fluoride.
-
Decomposition: PF5 decomposes at high temperatures, releasing toxic fumes.
-
Coordination Chemistry: PF5 forms coordination complexes with various ligands.
-
Research Tool: It is a valuable tool in research for studying fluorine chemistry.
-
Historical Use: PF5 was historically used in the production of certain pesticides.
-
Regulations: Handling and usage of PF5 are regulated due to its hazardous nature.
Phosphorus Pentafluoride: Key Takeaways
Phosphorus pentafluoride, a fascinating compound, plays a crucial role in various chemical reactions. Known for its high reactivity and unique properties, it’s used in organic synthesis and fluorination processes. This compound, with its pungent odor and colorless gas form, requires careful handling due to its toxicity and corrosive nature.
Understanding its chemical structure and applications helps in appreciating its significance in the chemical industry. From catalysts to intermediates, phosphorus pentafluoride proves indispensable in many fields.
By learning about its uses, safety measures, and environmental impact, we gain a comprehensive view of this intriguing substance. Whether you’re a chemistry enthusiast or a professional, knowing these facts enhances your knowledge and appreciation of phosphorus pentafluoride.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
