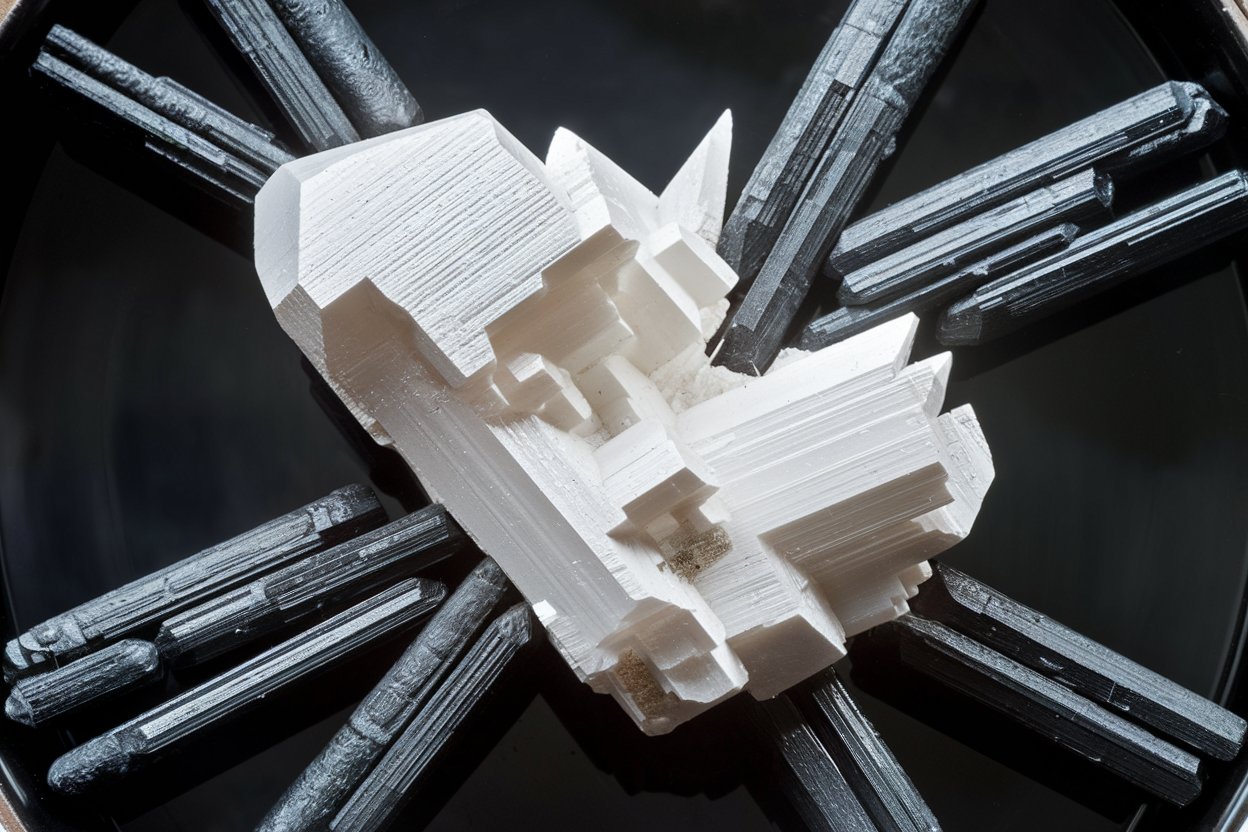
Barium oxalate might sound like a mouthful, but this compound holds some intriguing secrets. What is barium oxalate? Barium oxalate is a white, crystalline powder often used in pyrotechnics, ceramics, and even in certain chemical reactions. This compound, with the formula BaC2O4, combines barium and oxalic acid. It’s not just a lab curiosity; it has practical applications that make it quite valuable. From creating vibrant fireworks to serving as a precursor in various industrial processes, barium oxalate plays a significant role. Ready to dive into 40 fascinating facts about this compound? Let’s get started!
Key Takeaways:
- Barium oxalate, a white powder used in fireworks, has a toxic nature and unique chemical properties. Its historical and modern applications make it a fascinating compound for scientific and industrial use.
- Handling barium oxalate requires strict safety measures due to its toxicity. Its environmental impact and historical significance add depth to its intriguing presence in popular culture.
What is Barium Oxalate?
Barium oxalate is a chemical compound with the formula BaC₂O₄. It appears as a white powder and is known for its use in pyrotechnics and other applications. Let's dive into some fascinating facts about this compound.
-
Chemical Formula: The chemical formula for barium oxalate is BaC₂O₄, indicating it contains barium, carbon, and oxygen.
-
Appearance: It typically appears as a white crystalline powder.
-
Solubility: Barium oxalate is poorly soluble in water, making it less likely to dissolve easily.
-
Toxicity: This compound is toxic if ingested, inhaled, or comes into contact with skin.
-
Pyrotechnics: Barium oxalate is often used in fireworks to produce green colors.
Chemical Properties of Barium Oxalate
Understanding the chemical properties of barium oxalate can help us appreciate its various applications and safety measures.
-
Decomposition: When heated, barium oxalate decomposes into barium carbonate and carbon monoxide.
-
Reactivity: It reacts with strong acids to form oxalic acid and barium salts.
-
Molecular Weight: The molecular weight of barium oxalate is approximately 225.34 g/mol.
-
Melting Point: Barium oxalate has a melting point of around 400°C.
-
Density: The density of barium oxalate is about 2.658 g/cm³.
Uses of Barium Oxalate
Barium oxalate has several practical applications, especially in industries and scientific research.
-
Fireworks: It is used to create green colors in fireworks displays.
-
Ceramics: Barium oxalate is sometimes used in ceramic glazes.
-
Analytical Chemistry: It serves as a reagent in various chemical analyses.
-
Pigments: This compound is used in the production of certain pigments.
-
Laboratory Reagent: Barium oxalate is utilized as a reagent in laboratory experiments.
Safety and Handling of Barium Oxalate
Given its toxicity, handling barium oxalate requires strict safety measures to avoid health risks.
-
Protective Gear: Always wear gloves, goggles, and protective clothing when handling barium oxalate.
-
Ventilation: Ensure proper ventilation in areas where barium oxalate is used to avoid inhalation.
-
Storage: Store in a cool, dry place away from incompatible substances.
-
First Aid: In case of exposure, seek immediate medical attention and follow first aid procedures.
-
Disposal: Dispose of barium oxalate according to local environmental regulations.
Environmental Impact of Barium Oxalate
The environmental impact of barium oxalate is an important consideration, especially in industrial applications.
-
Water Contamination: Improper disposal can lead to water contamination, affecting aquatic life.
-
Soil Contamination: It can also contaminate soil, posing risks to plants and animals.
-
Air Pollution: Burning barium oxalate can release harmful gases into the atmosphere.
-
Biodegradability: Barium oxalate is not biodegradable, making it persistent in the environment.
-
Regulations: Many countries have regulations in place to control the use and disposal of barium oxalate.
Historical Facts about Barium Oxalate
The history of barium oxalate is filled with interesting developments and discoveries.
-
Discovery: Barium oxalate was first synthesized in the 19th century.
-
Early Uses: Initially, it was used in early pyrotechnic compositions.
-
Scientific Research: It has been the subject of numerous scientific studies over the years.
-
Industrial Adoption: The compound saw increased industrial use in the 20th century.
-
Modern Applications: Today, barium oxalate is used in various modern applications, from fireworks to ceramics.
Fun Facts about Barium Oxalate
Let's explore some fun and lesser-known facts about barium oxalate.
-
Color Change: When heated, it changes color due to decomposition.
-
Crystal Structure: Barium oxalate forms interesting crystal structures under certain conditions.
-
Magnetism: It is not magnetic, despite containing barium.
-
Luminescence: Some barium oxalate compounds exhibit luminescence under UV light.
-
Historical Fireworks: Ancient Chinese fireworks sometimes used barium oxalate for green colors.
Barium Oxalate in Popular Culture
Barium oxalate has even made its way into popular culture in surprising ways.
-
Movies: It has been referenced in movies featuring pyrotechnics.
-
Books: Some chemistry textbooks highlight barium oxalate in discussions on pyrotechnics.
-
TV Shows: Science shows occasionally feature barium oxalate in experiments.
-
Art: Some artists use barium oxalate in creating unique pigments.
-
Music: Believe it or not, some musicians have used barium oxalate in stage effects for concerts.
Final Thoughts on Barium Oxalate
Barium oxalate, a fascinating compound, has a lot to offer. From its role in pyrotechnics to its use in chemical analysis, this substance is more than just a name on the periodic table. Its unique properties make it valuable in various industries, including fireworks and ceramics. Understanding its characteristics can help in safely handling and utilizing it in different applications. Always remember, though, that safety comes first when dealing with chemicals. Proper storage and handling procedures are crucial to prevent any accidents. Whether you're a student, a hobbyist, or a professional, knowing these 40 facts about barium oxalate can deepen your appreciation for this versatile compound. Keep exploring and learning, and who knows what other interesting facts you might uncover next!
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
