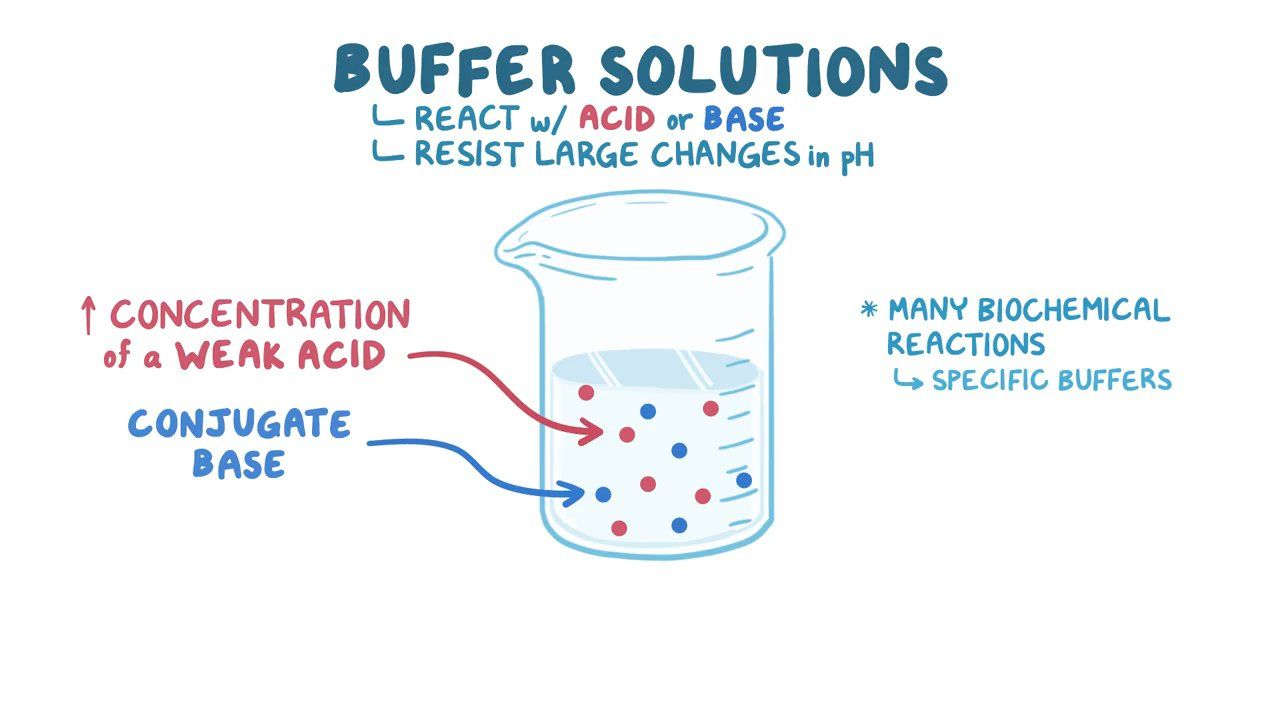
Buffer solutions are an essential aspect of chemistry that play a crucial role in maintaining pH levels in various chemical and biological systems. These solutions are a blend of weak acids or bases and their conjugate salts, designed to resist changes in pH when additional acid or base is introduced. While buffer solutions may seem like a simple concept, they hold a wide range of enigmatic facts and intricacies that make them fascinating to chemists and researchers alike.
In this article, we will delve into the intriguing world of buffer solutions and explore 20 enigmatic facts that shed light on their importance, applications, and underlying principles. From their ability to maintain a stable pH to their involvement in biological processes, we will uncover the mysteries surrounding these solutions and gain a deeper understanding of their significance in the world of chemistry.
Key Takeaways:
- Buffer solutions are like bodyguards for pH levels, keeping them stable and safe from sudden changes, making them crucial for experiments and even preserving historical artifacts.
- They are like magical potions made of weak acids and bases, helping maintain the pH balance in living organisms, pharmaceuticals, and even the food we eat!
Buffer solutions resist dramatic changes in pH levels.
A buffer solution is a carefully crafted mixture that helps maintain a stable pH level, even when an acid or base is added. It acts as a safeguard against sudden changes in the acidity or alkalinity of a solution.
The pH of a buffer solution remains relatively constant.
Buffer solutions are designed to resist changes in pH, making them ideal for various applications, such as biochemical experiments and medical laboratory procedures.
Buffer solutions are made up of a weak acid and its conjugate base, or a weak base and its conjugate acid.
By combining a weak acid and its conjugate base, or a weak base and its conjugate acid, a buffer solution can effectively maintain a stable pH range. This is due to the equilibrium between the weak acid and its conjugate base, which acts as a buffer against pH changes.
Buffer solutions are commonly used in biological and chemical experiments.
The use of buffer solutions is vital in biological and chemical research, as they provide a stable environment for delicate reactions and help maintain the integrity of biological samples.
Buffer solutions are essential in maintaining the pH balance of living organisms.
Living organisms have specific pH requirements for different physiological processes. Buffer solutions play a crucial role in regulating and maintaining the pH balance within living systems.
Buffer solutions can be prepared using various components.
Buffer solutions can be made by combining a weak acid or base with its conjugate salt, or by using a mixture of weak acids and bases with their respective conjugate salts. This allows for flexibility in preparing buffer solutions for different pH ranges.
Buffer solutions are used in the production of pharmaceutical drugs.
Buffer solutions play a critical role in pharmaceutical manufacturing, as they ensure that drugs remain stable and maintain their efficacy throughout the production process and storage.
Buffer solutions are utilized in DNA and protein research.
The study of DNA and proteins often requires a specific pH environment. Buffer solutions are used to create and maintain the optimal pH conditions necessary for DNA sequencing, protein analysis, and other molecular biology techniques.
Buffer solutions are employed in chromatography techniques.
In chromatography, buffer solutions are used to maintain a constant pH, which ensures accurate separation and analysis of different compounds within a mixture.
Buffer solutions are crucial in the food and beverage industry.
Buffer solutions are used in food and beverage processing to control and maintain the pH of products, ensuring their safety, quality, and stability.
Buffer solutions can be prepared with natural substances.
Some buffer solutions can be created using naturally occurring substances, such as citric acid and sodium citrate, making them environmentally friendly alternatives in certain applications.
Buffer solutions are widely used in analytical chemistry.
Buffer solutions are essential tools in analytical chemistry as they provide a consistent pH environment for accurate measurements and analyses.
Buffer solutions can be prepared with precise pH values.
By carefully selecting the appropriate weak acid and conjugate base, or weak base and conjugate acid, buffer solutions with specific pH values can be prepared, allowing for precise control in experiments and applications.
Buffer solutions can undergo changes in temperature.
Temperature changes can affect the pH stability of buffer solutions. It is important to consider temperature variations when using buffer solutions in different experimental conditions.
Buffer solutions are used in the preservation of historical artifacts.
Buffer solutions play a role in the preservation and restoration of historical artifacts by helping to maintain stable pH conditions, which can prevent deterioration and degradation.
Buffer solutions are essential in biological buffers.
Biological buffers are buffer solutions specifically designed to maintain a specific pH range for various biological processes, such as enzyme activity and cell culture.
Buffer solutions can extend the shelf life of certain products.
Buffer solutions can be incorporated into certain products to help maintain their stability and prolong their shelf life, ensuring that they remain effective over an extended period of time.
Buffer solutions can be used in water treatment processes.
Buffer solutions aid in maintaining the pH balance of water during treatment processes, ensuring that water remains safe for consumption and meets regulatory standards.
Buffer solutions are used in the calibration of pH meters.
Buffer solutions with known and stable pH values are used to calibrate pH meters, ensuring accurate and reliable measurements in various scientific and industrial settings.
Buffer solutions are an indispensable tool in chemical education.
Buffer solutions are frequently used in educational laboratories to teach principles of acid-base equilibrium, pH, and buffer systems in chemistry and related disciplines.
Conclusion
In conclusion, buffer solutions are an essential concept in the field of chemistry. They play a crucial role in maintaining the pH balance in various chemical processes, biological systems, and industrial applications. Understanding the properties and uses of buffer solutions is vital for scientists, researchers, and chemists alike.Buffer solutions provide a stable environment where the pH remains relatively constant, even when there are additions of acids or bases. This unique property is due to the presence of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer solutions find applications in various fields such as pharmaceuticals, agriculture, biochemistry, and environmental science.By utilizing buffer solutions, scientists can perform accurate and precise experiments, maintain optimal conditions for enzymatic reactions, and stabilize pH levels in biological systems. Buffer solutions are indispensable in laboratory settings and industrial processes, making them a key component in countless scientific advancements.In summary, buffer solutions are a significant aspect of chemistry and have revolutionized various scientific disciplines. Their ability to maintain pH stability has a profound impact on numerous applications, furthering our understanding and progress in the world of science.
FAQs
Q: What is a buffer solution?
A: A buffer solution is a solution that resists changes in pH when small amounts of acid or base are added to it. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
Q: What is the importance of buffer solutions?
A: Buffer solutions are important because they provide a stable pH environment, which is crucial for various chemical reactions, biological processes, and industrial applications.
Q: How are buffer solutions used in scientific research?
A: Buffer solutions are used in scientific research to maintain a constant pH, ensuring accurate and reproducible results in experiments.
Q: Can buffer solutions be used in everyday life?
A: Yes, buffer solutions can be found in everyday products such as antacids, shampoos, and cleaning agents. They help maintain the desired pH levels in these products.
Q: Are buffer solutions only used in chemistry?
A: No, buffer solutions have applications in various fields, including biochemistry, pharmaceuticals, agriculture, and environmental science.
Q: Can I create my own buffer solution?
A: Yes, buffer solutions can be prepared by mixing a weak acid and its conjugate base or a weak base and its conjugate acid in specific proportions.
Buffer solutions play a crucial role in maintaining stable pH levels, making them indispensable tools in various scientific and industrial applications. From preserving historical artifacts to ensuring the quality of pharmaceutical drugs, buffer solutions have proven their versatility and importance. As you've explored the enigmatic facts about buffer solutions, your curiosity might be piqued to learn more about related topics in chemistry. Why not delve into the fascinating world of amphoteric substances and uncover their unique properties?
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
