Denaturation is a fascinating process that occurs in the field of chemistry, and it holds numerous astounding facts that are worth exploring. From unraveling the structure of proteins to understanding the effects of heat and pH on biological molecules, denaturation plays a crucial role in various scientific endeavors. In this article, we will delve into 19 intriguing facts about denaturation that will amaze and captivate your curiosity. Whether you are a chemistry enthusiast or simply intrigued by the wonders of science, these facts will broaden your understanding of denaturation and its significance in our daily lives. So, let’s embark on this journey through the mysterious world of denaturation and uncover its bewildering secrets.
Key Takeaways:
- Denaturation is a process that changes the shape of proteins, affecting their function. It can happen due to heat, pH changes, or chemicals, and has big impacts on cooking, lab work, and even allergies.
- Denaturation isn’t just about proteins – it also affects DNA. It’s reversible for DNA, but not always for proteins. This process is super important in cooking, medicine, and understanding how our bodies work.
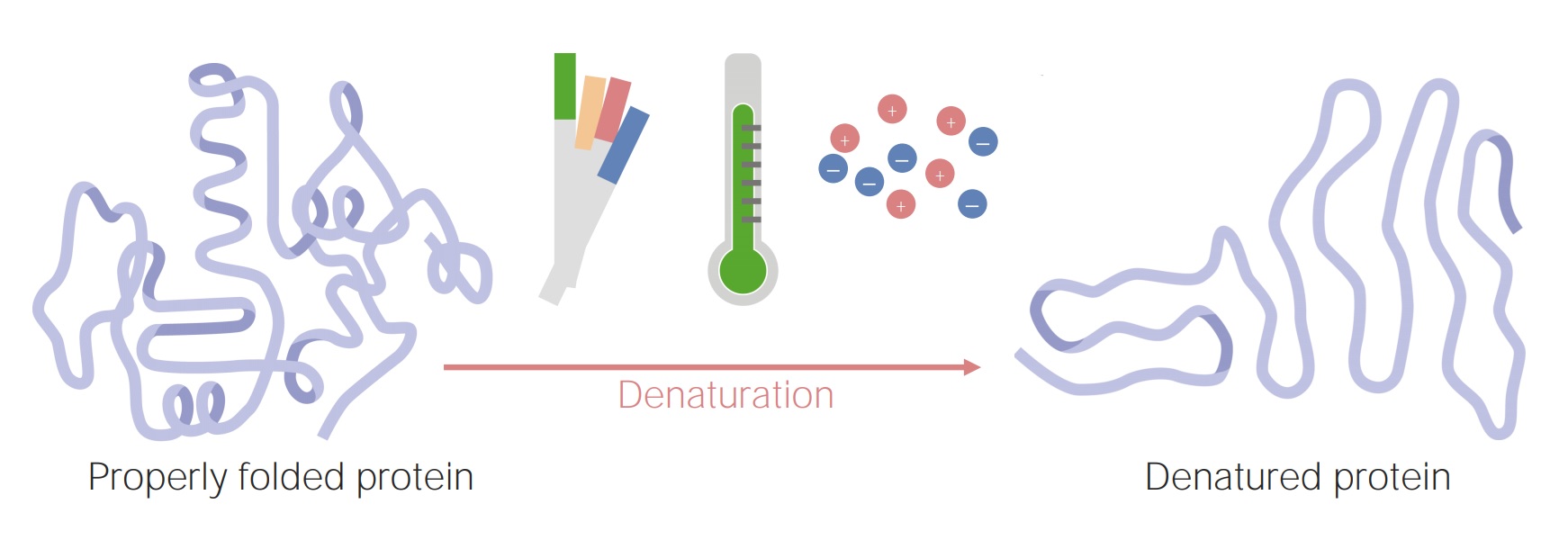
Denaturation is a process that alters the structure of proteins.
Denaturation refers to the disruption of the native conformation of proteins, resulting in a loss of their biological activity. This can occur due to various factors such as heat, pH changes, exposure to chemicals, or mechanical agitation.
Heat is a common cause of protein denaturation.
When proteins are exposed to high temperatures, the heat disrupts the weak bonds that hold the protein structure together, causing the protein to unfold and lose its functional shape. This denaturation process is irreversible in most cases.
Denaturation can also occur due to changes in pH.
Proteins have an optimal pH range at which they function best. Deviation from this pH range can lead to denaturation as the acidity or alkalinity disrupts the electrostatic interactions within the protein structure.
Chemicals like acids, bases, and salts can denature proteins.
Strong acids or bases can alter the charges on the amino acid residues of a protein, affecting their interactions and leading to denaturation. Similarly, certain salts can disrupt the balance of ions and disrupt the protein structure.
Denaturation can result in changes to the protein’s physical properties.
When a protein undergoes denaturation, it may lose its solubility in water, becoming insoluble. It may also change in color, texture, and taste, making it less appealing or useful in certain applications.
Denaturation can affect the biological activity of proteins.
Many proteins have specific functions in the body, such as enzymes that catalyze biochemical reactions or antibodies that target foreign invaders. Denaturation can impair these functions, rendering the protein inactive or less effective.
Denaturation is a reversible process in some cases.
While denaturation is often irreversible, some proteins can regain their native conformation under favorable conditions. This process is known as renaturation and involves restoring the protein’s original structure and functionality.
Denaturation can be used in food preparation.
Denaturation of proteins plays a crucial role in cooking processes such as the coagulation of egg whites or the browning of meat during grilling. These changes in protein structure contribute to the desired texture and flavor of cooked foods.
Denaturation is an essential step in certain laboratory techniques.
In molecular biology and biochemistry, denaturation is often employed to separate and isolate proteins or to study their structure and function. Techniques like SDS-PAGE or Western blotting rely on the denaturation of proteins for accurate analysis.
Denaturation can be reversible or irreversible depending on the conditions.
The extent of denaturation and the ability to renature a protein depend on the severity and duration of the denaturing conditions. Mild denaturing conditions might allow the protein to retain some of its structure, while severe denaturation can result in permanent loss of structure.
Denaturation can impact the allergenicity of proteins.
Some individuals may be allergic to specific proteins in their native forms. However, denaturation can alter the protein’s structure, making it unrecognizable by the immune system and reducing its allergenic potential.
Denaturation of DNA occurs when the double helix structure is disrupted.
Although denaturation is commonly associated with proteins, DNA molecules can also undergo denaturation. Heating or changes in pH can cause the DNA double helix to separate into single strands, disrupting the hydrogen bonds between nucleotide base pairs.
Denaturation can be reversible in DNA.
Unlike protein denaturation, DNA denaturation can be reversible. By adjusting the temperature or pH, the single strands of DNA can reanneal and reform the double helix structure.
Denaturation is important in DNA analysis techniques.
Denaturation plays a crucial role in techniques like polymerase chain reaction (PCR) and DNA sequencing. By heating the DNA strands to denature them, researchers can amplify specific DNA regions or determine the order of nucleotides.
Denaturation can affect the taste and nutritional quality of food.
The denaturation of proteins in food can alter their digestibility and affect the taste and texture of the final product. This is particularly important in the processing and cooking of proteins like meat, dairy products, and legumes.
Denaturation can be used in the production of biopharmaceuticals.
In the manufacturing of biopharmaceuticals such as insulin or antibodies, denaturation and subsequent renaturation steps are often employed to purify and stabilize the proteins, ensuring their safety and efficacy.
Denaturation can occur during protein folding.
Denaturation can happen when the process of protein folding goes awry, resulting in a misfolded or incorrectly formed protein structure. This can lead to the development of protein misfolding diseases like Alzheimer’s or Parkinson’s.
Denaturation can be visually detected in some cases.
When denaturation occurs, it may lead to visible changes in the appearance of the protein or the sample. For example, the coagulation or curdling of milk is a visible sign of protein denaturation.
Denaturation is a fundamental concept in biochemistry and biotechnology.
The study of denaturation provides insights into the structure, function, and stability of proteins. Understanding denaturation is crucial for designing drugs, developing new biotechnological processes, and exploring the dynamics of macromolecules in various environments.
Conclusion
Denaturation is a fascinating process that occurs in the field of chemistry. It involves the alteration of a protein’s structure, resulting in the loss of its biological activity. Through various factors such as temperature, pH level, and exposure to chemicals, denaturation can occur, changing the properties and function of proteins.In this article, we have explored 19 astounding facts about denaturation. We have learned about the different factors that can lead to denaturation, such as heat and chemical agents. We have also discovered the impact of denaturation on various biological processes, from cooking and digestion to the unfolding of proteins.Understanding denaturation is crucial in fields like biochemistry, food science, and pharmaceuticals. It allows scientists to study and manipulate protein structures to develop new drugs, optimize food production, and uncover the mysteries of various biological processes.Denaturation is a complex yet captivating phenomenon that continues to intrigue scientists worldwide. By delving deeper into its intricacies, we can unlock new knowledge and pave the way for groundbreaking discoveries in the field of chemistry.
FAQs
1. What is denaturation in chemistry?
Denaturation is the process through which the structure of a protein is altered, leading to the loss of its biological activity.
2. What factors can cause denaturation?
Denaturation can be caused by various factors such as extreme temperatures, changes in pH levels, exposure to chemicals, and mechanical agitation.
3. How does denaturation affect proteins?
Denaturation disrupts the protein’s native structure, leading to the loss of its biological function. The protein may unfold or aggregate, rendering it inactive or even harmful.
4. What are some examples of denaturation?
Cooking an egg, where the heat causes the proteins in the egg white to denature, is a commonly observed example of denaturation. Another example is the denaturation of enzymes at high temperatures, which can lead to a loss of enzyme activity.
5. Can denaturation be reversed?
In some cases, denaturation can be reversed, especially if the protein is not irreversibly damaged. However, in many instances, denaturation is irreversible, and the protein’s original function cannot be restored.
6. How is denaturation important in the food industry?
Denaturation plays a crucial role in cooking processes, such as baking, grilling, and frying, where proteins in food undergo structural changes leading to desirable taste, texture, and color.
7. How does denaturation impact pharmaceutical development?
In the field of pharmaceuticals, denaturation helps scientists understand how proteins interact with drugs, aiding in the design and development of effective medications.
Unraveling denaturation's mysteries is just the beginning of your scientific journey. Dive deeper into life's building blocks with molecular biology facts that will leave you in awe. Explore chemical reactions' intricacies by understanding reaction coordinate's captivating truths. Fuel your body and mind with protein-packed chocolate powder nutrition facts. Embark on a knowledge-filled adventure that satisfies your curiosity and appetite for learning.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
