Geometric isomerism is a fascinating concept within the field of chemistry that can leave even the most seasoned chemists in awe. It involves the arrangement of atoms and groups of atoms around a double bond or a chiral center, resulting in different spatial arrangements of molecules. These subtle variations can have profound implications on the physical and chemical properties of compounds.
In this article, we will delve into the intriguing world of geometric isomerism and explore 18 unbelievable facts that will broaden your understanding of this phenomenon. From the mind-boggling optical isomerism to the puzzling cis-trans isomerism, we will uncover the hidden secrets behind these molecular structures and how they impact our everyday lives.
So, buckle up and prepare to be amazed as we journey through the astonishing world of geometric isomerism!
Key Takeaways:
- Geometric isomerism is like a molecular puzzle, where atoms are arranged differently in space, leading to unique properties and behaviors in compounds. It’s like a secret code that changes how molecules act!
- Just like a secret language, geometric isomerism affects how drugs work in our bodies and influences the complexity of chemical reactions. It’s like a hidden superpower that scientists use to create new medicines and materials!
Geometric isomerism refers to the arrangement of atoms in molecules.
Geometric isomerism occurs when two or more compounds have the same molecular formula, but their atoms are arranged differently in space.
It is a type of stereoisomerism.
Geometric isomerism falls under the broader category of stereoisomerism, which describes compounds with the same connectivity of atoms but different spatial arrangements.
Geometric isomers have different physical and chemical properties.
Due to their distinct spatial arrangements, geometric isomers often exhibit variations in boiling points, melting points, solubilities, and reactivity.
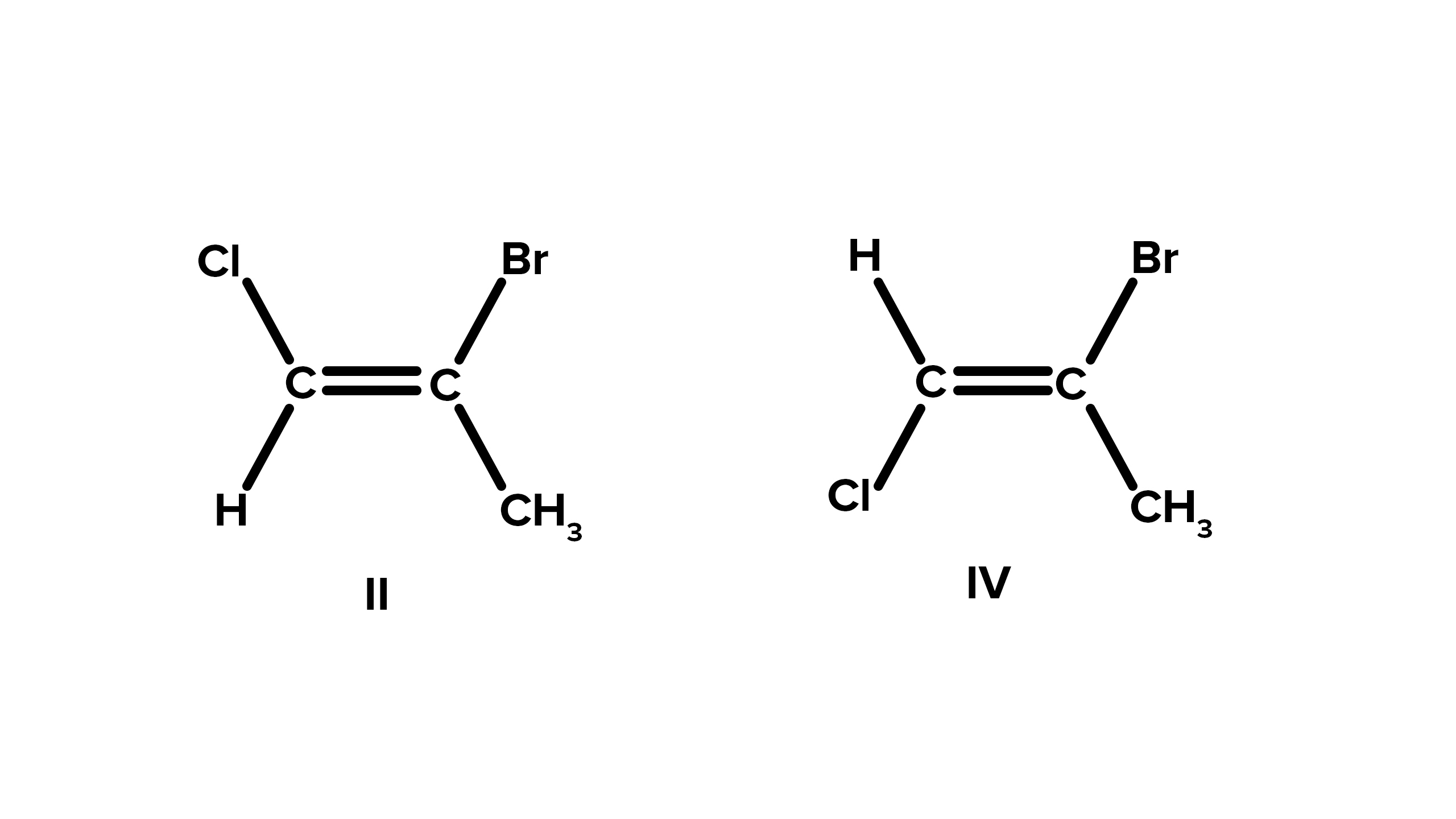
Cis-trans isomerism is a common form of geometric isomerism.
In cis-trans isomerism, the isomers differ in the placement or positioning of atoms or groups on opposite sides of a rigid bond.
It can occur in compounds with double bonds.
Geometric isomerism is commonly observed in molecules with double bonds, where restricted rotation about the bond allows different arrangements of atoms.
Geometric isomerism can affect biological activity.
Due to their distinct structures, geometric isomers can display different biological properties, affecting factors such as drug effectiveness or toxicity.
It is relevant in organic chemistry.
Geometric isomerism often plays a crucial role in organic chemistry, influencing reactions, compound stability, and synthesis strategies.
Geometric isomers have different spatial arrangements.
In geometric isomerism, the compounds have distinct arrangements of atoms in three-dimensional space, leading to different physical properties and behaviors.
The isomers are not interconvertible under ordinary conditions.
Unlike other forms of isomerism, geometric isomers cannot be converted into each other without breaking or re-forming bonds.
It can occur in cyclic compounds.
Cyclic compounds can also exhibit geometric isomerism, where the orientation of substituents around the ring differs in different isomers.
Geometric isomerism can be visualized using Newman projections.
Newman projections are often used to illustrate different conformations and geometric isomers of organic molecules.
It is important in the study of coordination compounds.
Geometric isomerism is crucial in understanding the behavior and properties of coordination compounds, which are widely used in various applications.
Different geometric isomers may have different biological activity.
In the field of medicinal chemistry, the geometric isomerism of certain compounds can significantly influence their biological activity and interactions with targets in the body.
Geometric isomerism contributes to the complexity of chemical reactions.
The presence of geometric isomers in a reaction mixture can lead to diverse reaction pathways and product formations, adding complexity to chemical processes.
It can be observed in coordination isomerism.
Coordination isomerism, a type of geometrical isomerism, occurs when the arrangement of ligands around the central metal ion differs in different isomeric compounds.
Geometric isomerism can affect the stability of compounds.
Geometric isomers may have varying levels of stability due to differences in molecular strain or the presence of steric hindrance between substituents.
It is an important concept in understanding the structure-activity relationship of molecules.
The study of geometric isomerism helps scientists comprehend how the arrangement of atoms affects the properties and activities of molecules, aiding in the design of new drugs and materials.
Geometric isomerism can be detected and analyzed using various spectroscopic techniques.
Spectroscopic methods such as infrared spectroscopy and NMR spectroscopy are often employed to identify and characterize geometric isomers in chemical compounds.
Conclusion
Geometric isomerism is a fascinating aspect of chemistry that involves the spatial arrangement of atoms in molecules. Through the exploration of these 18 unbelievable facts about geometric isomerism, we have delved into the intricacies of how isomers differ in their arrangement and properties.
We discovered that geometric isomerism arises due to the restricted rotation around double bonds or the presence of a ring structure in a molecule. This phenomenon can have profound effects on the physical and chemical properties of substances, leading to variations in their biological activity, stability, and reactivity.
Understanding geometric isomerism has numerous practical applications in fields like pharmaceuticals, agriculture, and materials science. By manipulating the arrangement of atoms, chemists can optimize drug efficacy, design better crop protection agents, and develop new materials with tailored properties.
As our knowledge of geometric isomerism continues to grow, so does our appreciation for the complexity and beauty of the molecular world. This field of study will undoubtedly continue to unlock new possibilities and drive advancements in various scientific disciplines.
FAQs
Q: What is geometric isomerism?
A: Geometric isomerism refers to the phenomenon where molecules have the same molecular formula and connectivity of atoms but differ in spatial arrangement due to restricted rotation around double bonds or the presence of a ring structure.
Q: How does geometric isomerism affect the properties of molecules?
A: Geometric isomerism can result in distinct physical and chemical properties in molecules. This can include differences in melting and boiling points, solubility, stability, reactivity, and biological activity.
Q: What causes geometric isomerism?
A: Geometric isomerism occurs due to the presence of multiple bonds or ring structures that prevent free rotation around the carbon-carbon bond, leading to different spatial arrangements.
Q: What are some examples of geometric isomers?
A: Examples of geometric isomers include cis-trans isomerism in alkenes, where substituents are either on the same or opposite sides of the double bond, and cis-trans isomerism in cyclic compounds, where substituents are either on the same or opposite sides of the ring.
Q: What are the practical applications of understanding geometric isomerism?
A: Understanding geometric isomerism has applications in pharmaceuticals, agriculture, and materials science. It allows chemists to optimize drug efficacy, design better crop protection agents, and develop materials with specific properties.
Q: Can geometric isomerism occur in other elements besides carbon?
A: Yes, geometric isomerism can occur in compounds containing other elements like sulfur, nitrogen, and phosphorus. In these cases, the arrangement of atoms around the central atom determines the isomeric forms.
Geometric isomerism's impact on molecular structures, properties, and real-world applications is truly fascinating. Understanding how atoms arrange themselves in space can help us grasp the complexities of molecular geometry and its influence on chemical behavior. Equally intriguing is the world of optical isomerism, where mirror-image molecules exhibit unique characteristics that have far-reaching implications in fields like pharmaceuticals and materials science. By exploring these captivating aspects of chemistry, we can gain a deeper appreciation for the intricate dance of atoms and the profound effects they have on our lives.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
