Degenerate orbitals are a fascinating aspect of chemistry that play a significant role in understanding the behavior of electrons within atoms and molecules. These unique orbitals exhibit a range of extraordinary properties and characteristics that set them apart from other types of orbitals. In this article, we will explore 18 intriguing facts about degenerate orbitals that will deepen your understanding of this captivating subject. From their role in determining electronic configurations to their impact on chemical bonding, degenerate orbitals have a profound influence on the properties and reactivity of different chemical systems. So, let’s dive into the world of degenerate orbitals and uncover the amazing features that make them so remarkable.
Key Takeaways:
- Degenerate orbitals are like energy twins for electrons, influencing bonding and electron behavior in atoms and molecules. They’re like the secret agents of chemistry, shaping the world of chemical reactions and materials science.
- Quantum chemistry detectives study degenerate orbitals to unlock the secrets of electron configurations, molecular symmetry, and even the colors of transition metal complexes. It’s like solving a fascinating puzzle to understand the building blocks of our world.
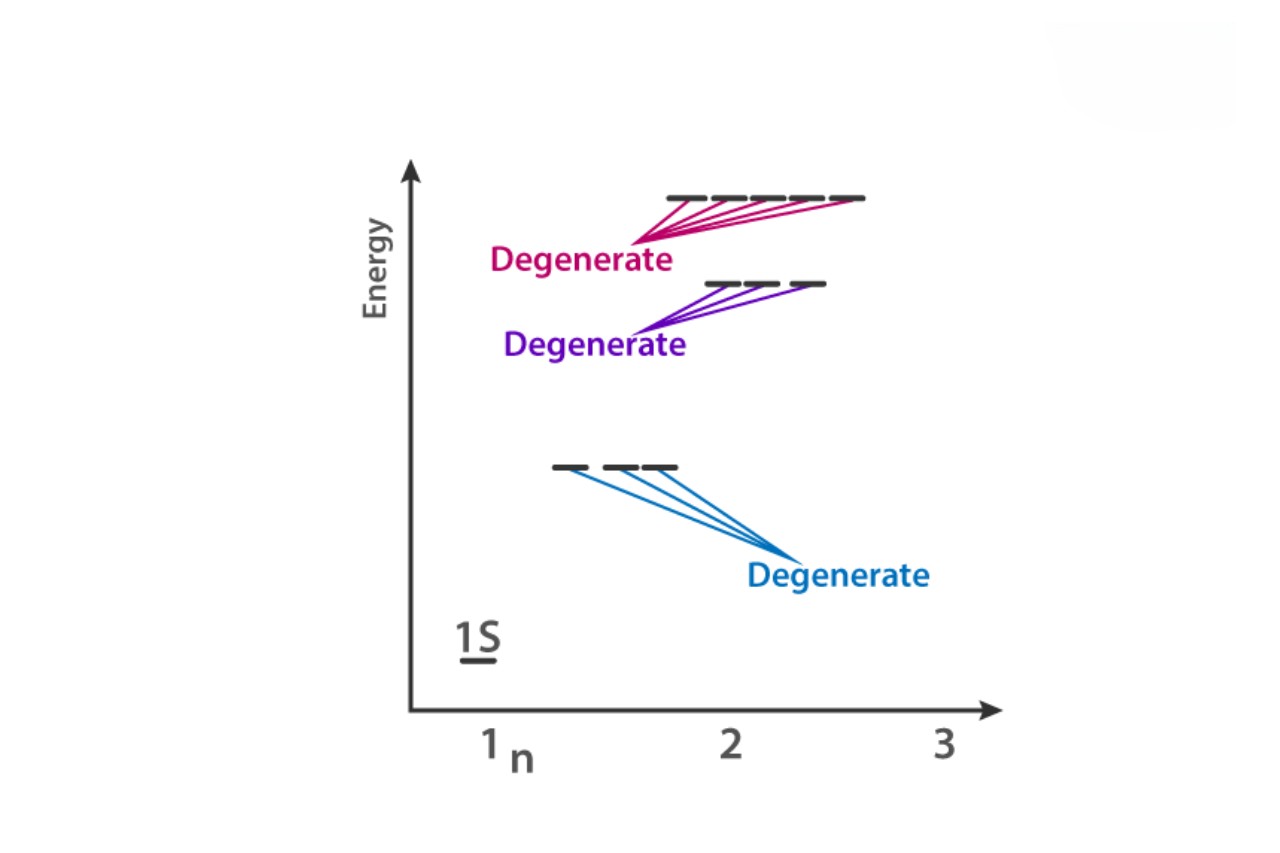
Degenerate orbitals are energy levels that have the same energy.
Degenerate orbitals refer to atomic orbitals that have the same energy level. This means that electrons occupying degenerate orbitals have equal energy and are therefore indistinguishable from one another.
Degenerate orbitals are found in atoms with multiple electrons.
Degenerate orbitals typically occur in atoms with more than one electron. This is because the interactions between electrons in these atoms lead to the splitting of energy levels, resulting in degenerate orbitals with the same energy.
Degenerate orbitals play a key role in chemical bonding.
Degenerate orbitals are crucial in determining the bonding behavior of atoms. When atoms form chemical bonds, the degenerate orbitals can overlap and interact, resulting in the formation of molecular orbitals.
Degenerate orbitals can have different shapes and orientations.
Although degenerate orbitals share the same energy level, they can have different shapes and orientations. For example, in the p orbital set, the px, py, and pz orbitals are degenerate but have different spatial orientations.
Degenerate orbitals can be labeled using quantum numbers.
Quantum numbers are used to label degenerate orbitals and differentiate them from one another. These numbers include the principal quantum number (n), azimuthal quantum number (l), and magnetic quantum number (m).
Degenerate orbitals determine the electron configurations of atoms.
The arrangement of electrons in degenerate orbitals determines the electron configuration of an atom. This configuration plays a crucial role in determining the chemical properties and behavior of elements.
Degenerate orbitals can be visualized using atomic orbital diagrams.
Atomic orbital diagrams are used to represent degenerate orbitals and their electron occupancy. These diagrams show the energy levels, the shape of the orbitals, and the arrangement of electrons within the orbitals.
Degenerate orbitals can interact differently with external magnetic fields.
When exposed to external magnetic fields, degenerate orbitals can split into multiple energy levels. This phenomenon, known as the Zeeman effect, occurs due to the different orientations of the orbital’s magnetic moments.
Degenerate orbitals can influence the color of transition metal complexes.
Transition metal complexes with degenerate d orbitals can absorb certain wavelengths of light, resulting in the observed color of the complex. This phenomenon is known as ligand field theory and is utilized in various fields including materials science and biochemistry.
Degenerate orbitals are essential for understanding electron configurations in polyatomic molecules.
When considering polyatomic molecules, the presence of degenerate orbitals becomes crucial in understanding the distribution of electrons among the constituent atoms. This information helps predict the stability and reactivity of these molecules.
Degenerate orbitals can be calculated using quantum mechanical models.
Theoretical models based on quantum mechanics can be used to calculate and predict the properties of degenerate orbitals. These models, such as the Schrödinger equation, allow researchers to understand the behavior of electrons in atoms and molecules.
Degenerate orbitals can give rise to molecular symmetry.
The presence of degenerate orbitals in a molecule contributes to its overall symmetry. The symmetry of a molecule influences its physical properties, reactivity, and behavior in chemical reactions.
The number of degenerate orbitals depends on the electron configuration.
The number of degenerate orbitals present in an atom or molecule depends on the specific electron configuration. Different configurations result in different numbers and types of degenerate orbitals.
Degenerate orbitals can lead to the formation of hybrid orbitals.
In certain cases, degenerate orbitals can undergo hybridization, resulting in the formation of hybrid orbitals. These hybrid orbitals have specific shapes and orientations, which play a crucial role in molecular bonding and geometry.
Degenerate orbitals provide insights into electron spin.
Electrons occupying degenerate orbitals have opposite spins, following the Pauli exclusion principle. This spin information is crucial in understanding chemical bonding and electron behavior within atoms and molecules.
Degenerate orbitals are important in understanding electronic transitions and spectroscopy.
When electrons transition between energy levels, the presence of degenerate orbitals influences the wavelength and intensity of electromagnetic radiation absorbed or emitted. Spectroscopic techniques utilize this information to study the electronic structure of molecules.
Degenerate orbitals are widely studied in the field of quantum chemistry.
Quantum chemists extensively study degenerate orbitals to understand the behavior and properties of atoms and molecules. These studies contribute to advancements in fields such as materials science, drug discovery, and environmental chemistry.
Degenerate orbitals can lead to the phenomenon of electron delocalization.
In molecules with degenerate orbitals, electrons can be delocalized over multiple atoms or regions. This delocalization contributes to the stability and distinctive properties of these molecules, such as aromaticity in benzene.
In conclusion, degenerate orbitals play a critical role in atomic and molecular systems, influencing bonding, electronic structure, and spectroscopic properties. Understanding the nature and characteristics of degenerate orbitals contributes to various scientific disciplines and facilitates technological advancements.
Conclusion
In conclusion, degenerate orbitals are a fascinating aspect of chemistry that play a crucial role in understanding the behavior of electrons in atoms. These orbitals provide valuable insights into electron configurations, molecular bonding, and spectroscopic phenomena. By exploring these 18 extraordinary facts about degenerate orbitals, we have gained a deeper understanding of their significance and relevance in the world of chemistry.Degenerate orbitals exhibit several unique characteristics, such as identical energies, distinct shapes, and varying orientations. They contribute to the complex nature of chemical reactions and offer scientists a valuable tool for predicting and explaining the behavior of molecules. Whether it’s in quantum mechanics or organic chemistry, degenerate orbitals continue to intrigue researchers and unlock new avenues of knowledge.As we continue to study and unravel the mysteries of degenerate orbitals, we can expect further advancements in various fields of chemistry, leading to innovative technologies and groundbreaking discoveries. Embracing these extraordinary orbitals and their intricacies will undoubtedly shape the future of scientific exploration and enhance our understanding of the world around us.
FAQs
1. What are degenerate orbitals?
Degenerate orbitals are a set of orbitals in an atom or molecule that possess the same energy level.
2. How are degenerate orbitals formed?
Degenerate orbitals are formed when two or more atomic orbitals of the same energy level combine to create a new set of orbitals with the same energy.
3. What is the significance of degenerate orbitals?
Degenerate orbitals are crucial in determining electronic configurations, molecular bonding, and spectroscopic properties, providing valuable insights into chemical reactions and molecular behavior.
4. Are degenerate orbitals only found in atoms?
No, degenerate orbitals can also be observed in molecules, where the combination of atomic orbitals leads to the formation of molecular orbitals with the same energy.
5. Can degenerate orbitals have different shapes?
Yes, degenerate orbitals can have different shapes and orientations, depending on the combination of atomic orbitals from which they are formed.
6. How do degenerate orbitals influence bonding?
Degenerate orbitals can contribute to the formation of strong covalent bonds, as electrons occupying these orbitals can overlap with the orbitals of other atoms, leading to stable molecular structures.
7. Can degenerate orbitals be involved in chemical reactions?
Absolutely. Degenerate orbitals often play a crucial role in chemical reactions, influencing the reactivity and behavior of molecules during various chemical processes.
Degenerate orbitals fascinate chemists, physicists, students alike. Want to learn more? Dive into quantum chemistry and explore mind-boggling concepts. Uncover secrets of atomic structure, from electron configurations to spectroscopic transitions. Discover how degenerate orbitals influence molecular orbitals, shaping chemical bonding, symmetry, hybridization. Embark on a quantum journey that will leave you in awe of the subatomic world.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
