Chelate compounds have long been studied and utilized in the field of chemistry for their unique properties and applications. These fascinating compounds, formed by the bonding of a metal ion with multiple ligand molecules, play a crucial role in various chemical reactions and biological processes.
In this article, we will delve into the world of chelates and explore their intriguing characteristics. From their historical significance to their wide-ranging uses in fields such as medicine, agriculture, and environmental science, chelates have attracted attention from both researchers and industries alike.
So, buckle up and prepare to be amazed by the 16 fascinating facts about chelate compounds that will shed light on their importance and versatility in the world of chemistry.
Key Takeaways:
- Chelation enhances metal stability and plays a vital role in medicine, agriculture, and water treatment, making it a fascinating and versatile chemical process with wide-ranging applications.
- Chelation forms stable complexes, removes toxins, and influences biological processes, showcasing its importance in preserving cultural artifacts and advancing scientific research.
The Definition of Chelate
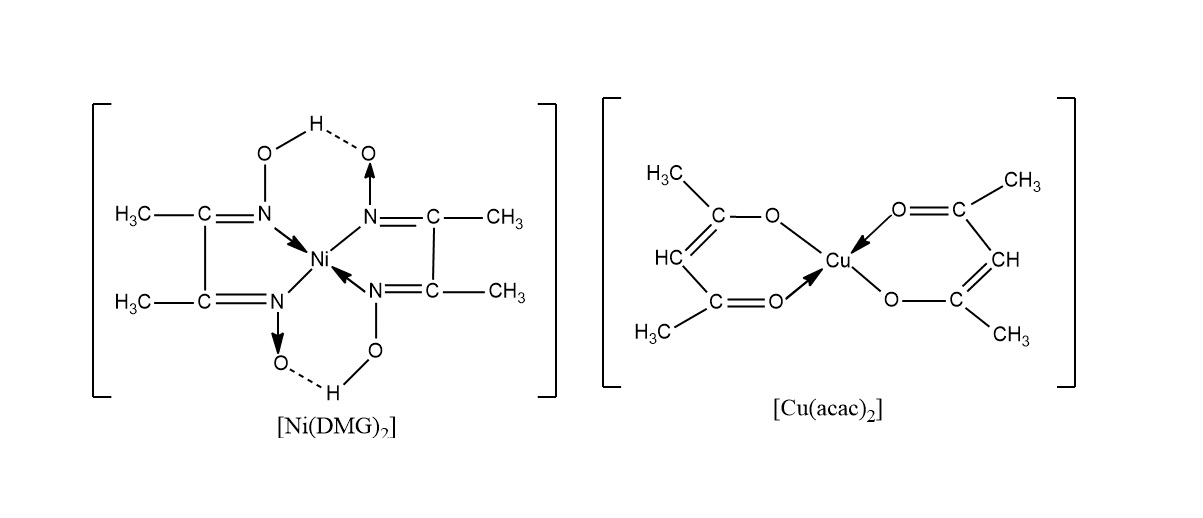
Chelate refers to a complex compound formed when a metal ion binds to a larger molecule by coordinating with multiple ligands. These ligands are usually organic compounds or ions known as chelating agents. The word “chelate” comes from the Greek word “chele,” meaning claw, which aptly depicts the way the ligands surround and grasp the metal ion. This process creates a stable and soluble complex that has various applications in chemistry, biology, and industry.
Chelate Formation Enhances Metal Stability
One fascinating fact about chelation is that it enhances the stability of metal ions. Metal ions alone tend to form insoluble precipitates or react with other substances in the environment, making them less useful for certain applications. However, when these metal ions chelate with appropriate ligands, they become more resistant to chemical changes, ensuring their efficacy and availability for specific purposes.
The Role of Chelation in Biochemistry
Chelation plays a crucial role in various biological processes. In biological systems, metal ions such as iron, copper, and zinc often form complexes with proteins to perform essential functions. These metalloproteins, as they are called, utilize the chelation process to coordinate with ligands and create active sites that facilitate enzymatic reactions, transport ions across cell membranes, and store or utilize metal ions for metabolic processes.
Applications of Chelation in Medicine
Chelation therapy is a medical treatment that utilizes chelating agents to remove heavy metal toxins from the body. Through a process called sequestration, the chelating agent forms stable complexes with the toxic metals, allowing them to be excreted from the body through urine or feces. This therapy is commonly used in cases of heavy metal poisoning or certain medical conditions, such as iron overload disorders.
The Use of Chelation in Agriculture
Chelate compounds are widely used in agriculture to improve the availability of essential trace elements like iron, zinc, manganese, and copper to plants. These compounds, known as chelated micronutrients, help prevent nutrient deficiencies, enhance plant growth, and increase crop yields. Chelated forms of essential nutrients are more readily absorbed by plant roots and are less prone to reacting with other compounds in the soil, ensuring efficient utilization by plants.
Chelation and Water Treatment
In water treatment, chelating agents are used to control and remove metal ions that can cause undesirable effects such as scaling, corrosion, or the formation of precipitates. Chelators bind to these metal ions, preventing them from reacting with other compounds or forming deposits. This process helps maintain the quality of water in various industries, including manufacturing, cooling systems, and wastewater treatment.
Chelation and Industrial Processes
The formation of chelate complexes is often exploited in industrial processes. Chelating agents are used to facilitate metal extraction from ores, purification of metals, and as catalysts in chemical reactions. The ability of chelators to selectively bind to specific metal ions is particularly valuable in these applications, allowing for efficient separation and purification processes.
Chelation and Analytical Chemistry
Chelation has significant implications in analytical chemistry, particularly in the determination and quantification of metal ions in various samples. Chelating agents can be used to selectively extract and concentrate specific metal ions from complex mixtures, making their analysis more accurate and reliable. This technique is commonly employed in environmental monitoring, forensic analysis, and quality control processes.
The Formation of Chelate Rings
Another interesting aspect of chelation is the formation of chelate rings. Some ligands are capable of coordinating with a metal ion at two or more binding sites, resulting in the formation of a cyclic structure. These chelate rings confer additional stability to the complex and often exhibit unique properties not observed in linear complexes. This unique feature allows for the design and development of new materials with specific applications in catalysis, sensors, and molecular electronics.
Chelation in Coordination Polymers
Coordination polymers, also known as metal-organic frameworks (MOFs), are hybrid materials consisting of metal ions coordinated to organic ligands. The chelation process plays a crucial role in the construction of these extended architectures, providing stability and directing the assembly of the network. Coordination polymers have gained significant attention due to their potential applications in gas storage, drug delivery systems, and catalysis.
Chelation as a Tool in Chemical Synthesis
Chelation is commonly used as a synthetic tool in organic chemistry. The introduction of chelating groups during chemical reactions can influence the selectivity, reactivity, and efficiency of the process. Chelation control is particularly useful in metal-catalyzed reactions, allowing for regioselective and stereoselective transformations. This strategy enables the synthesis of complex molecules with high precision and predictability.
The Role of Chelation in Photographic Film
In the production of photographic film, chelating agents are employed to remove metal impurities that can interfere with the image quality. These agents chelate with metal ions present in the film emulsion, preventing undesired reactions that could lead to image degradation or discoloration. Chelation helps ensure the production of high-quality photographic prints with accurate colors and enhanced longevity.
Chelation and Environmental Remediation
Chelating agents have been extensively studied for their potential applications in environmental remediation. These compounds can assist in the removal of heavy metals from contaminated sites, preventing their migration into groundwater or affecting ecosystems. Chelation-based remediation processes aim to sequester metal pollutants and immobilize them, reducing their toxicity and environmental impact.
Natural Chelators in Biological Systems
While synthetic chelating agents are widely used, it is worth mentioning that nature has its own versions of chelators. Many organisms produce natural chelators, such as siderophores, to bind and transport essential metals. Siderophores are small molecules secreted by bacteria, fungi, and plants to scavenge iron from the environment and help facilitate its uptake. Understanding these natural chelation mechanisms can inspire the development of new strategies in medication, agriculture, and environmental science.
Chelation in the Preservation of Cultural Artifacts
Chelating agents find valuable applications in the preservation and restoration of cultural artifacts and artworks. These agents can be used to remove metal contaminants or stabilize metal components that are susceptible to degradation. Chelation-based treatments help protect and extend the lifespan of historical objects, ensuring their cultural heritage is preserved for future generations.
Chelation as a Research Tool
Lastly, chelation serves as an essential tool in scientific research, allowing scientists to study metal interactions and unravel complex biological processes. By designing specific ligands to chelate with target metal ions, researchers can manipulate and control these interactions, shedding light on metal-dependent biological pathways, enzyme mechanisms, and disease processes.
These 16 fascinating facts about chelate highlight the diverse applications, importance, and intriguing features of this chemical phenomenon. From its role in medicine and agriculture to its significance in industrial processes and research, chelation continues to shape our understanding and improve various aspects of our lives.
Conclusion
In conclusion, chelate is a fascinating and important concept in chemistry. It involves the formation of coordination compounds where a metal ion is bonded to a ligand through multiple coordinating atoms. This unique bonding allows for enhanced stability, reactivity, and solubility of the complex. Chelation therapy, which utilizes chelating agents to remove heavy metals from the body, is an application of chelation that has gained significant attention in the medical field. Chelation also plays a crucial role in various industrial processes and environmental remediation. Understanding the intricacies of chelation can provide valuable insights into the behavior of complex chemical systems and pave the way for advancements in various scientific disciplines.
FAQs
1. What is chelate?
Chelate refers to the formation of a complex compound where a metal ion is bonded to a ligand through multiple coordinating atoms.
2. How does chelation therapy work?
Chelation therapy involves the use of chelating agents that bind to heavy metals in the body, helping to remove them and restore normal cellular function.
3. What are some examples of chelating ligands?
Some common examples of chelating ligands include ethylenediaminetetraacetic acid (EDTA), diethylene triamine pentaacetic acid (DTPA), and 1,10-phenanthroline.
4. What are the applications of chelation in industry?
Chelation finds applications in various industrial processes, such as metal extraction, catalysis, and as stabilizers in the production of detergents and cosmetics.
5. Is chelation only limited to metal ions?
No, chelation can also occur between an organic molecule and a metal ion or between two organic molecules, forming a coordination complex.
Chelation's diverse applications, from medicine to environmental remediation, demonstrate its significance in various fields. Complexometric titration, another fascinating chemical process, offers precise quantitative analysis of metal ions. Exploring complexometric titration reveals intriguing insights into analytical chemistry techniques. Unraveling complexometric titration's principles and applications deepens understanding of this valuable tool in laboratories worldwide. Delving into complexometric titration's nuances showcases its elegance and utility in quantifying metal content across industries.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
