Polarizability is a fundamental concept in the field of chemistry, which plays a crucial role in understanding the behavior of molecules and materials. It refers to the ability of a molecule to generate an electric dipole moment in response to an external electric field. The property of polarizability is not only important in explaining various chemical phenomena but also has practical applications in areas such as material science and drug design.
In this article, we will explore 10 fascinating facts about polarizability. From its definition and measurement methods to its impact on chemical reactivity and physical properties, we will delve into the intriguing world of polarizability. So, get ready to dive into the realm of chemical bonding and discover the intriguing aspects of this essential concept.
Key Takeaways:
- Polarizability measures how easily molecules can be distorted by an electric field, influencing their size, reactivity, and even their interactions with light and biological systems.
- Understanding polarizability helps scientists design new materials, develop advanced technologies, and even improve drug development and environmental chemistry. It’s a key factor in shaping the behavior and properties of molecules.
What is Polarizability?
Polarizability is a fundamental property of molecules that measures their ability to form temporary dipoles in response to an external electric field. It determines how easily the electron cloud of a molecule can be distorted.
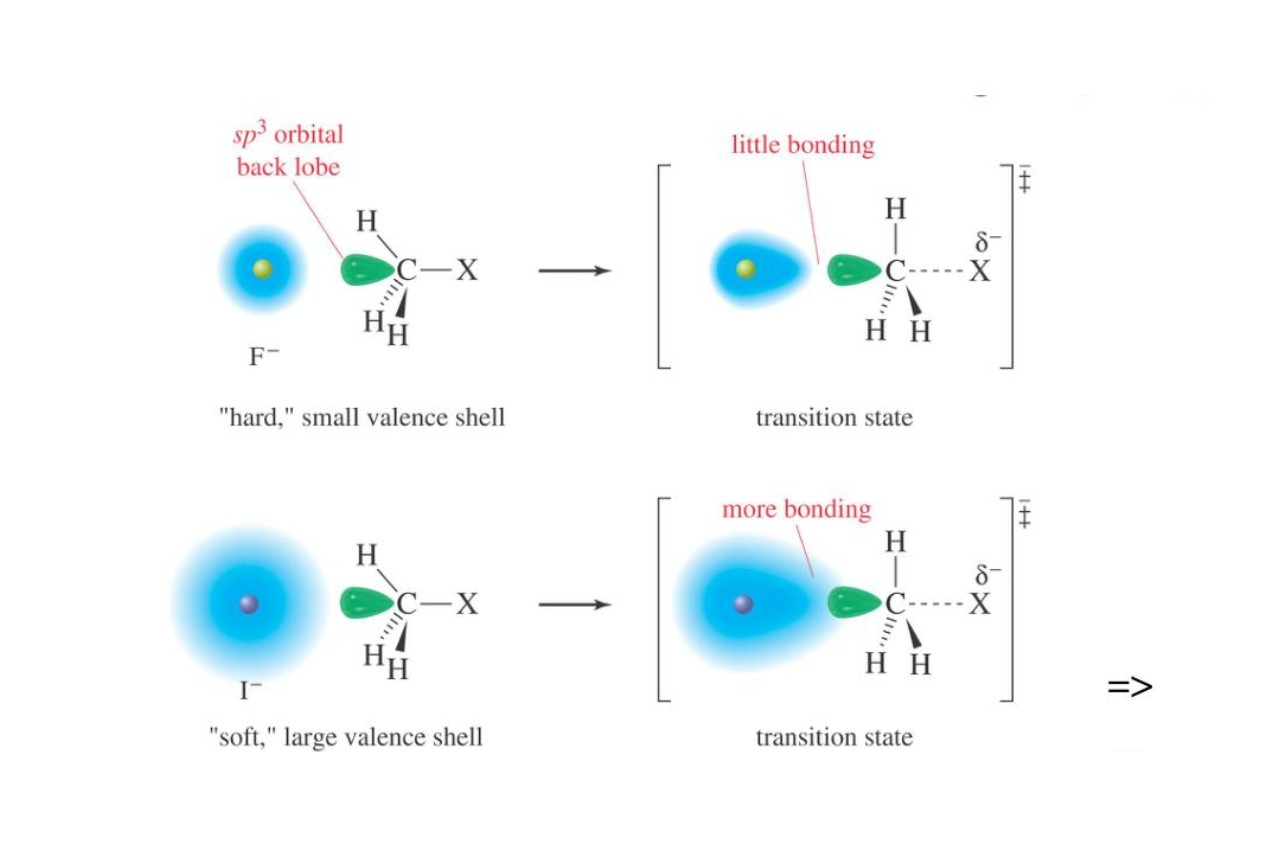
The Relationship Between Polarizability and Molecular Size
In general, larger molecules have higher polarizabilities due to the greater number of electrons and the larger electron cloud that can be distorted. This means that the ability of a molecule to be polarized increases as the size of the molecule increases.
Polarizability and Chemical Reactivity
The polarizability of a molecule plays a crucial role in its chemical reactivity. High polarizability can influence the strength and nature of intermolecular forces, solubility, and the ability to undergo chemical reactions.
Polarizability and Dispersion Forces
Dispersion forces, also known as London dispersion forces, arise due to temporary fluctuations in electron distribution within a molecule. The strength of these forces is directly related to the polarizability of the molecules involved.
Polarizability and Optical Properties
The polarizability of molecules also affects their optical properties. It determines how well a material can interact with light, influencing phenomena such as refraction, absorption, and scattering.
Polarizability and Dielectric Constant
The dielectric constant of a substance is a measure of its ability to store electrical energy in an electric field. Polarizability influences the dielectric constant, which has implications in various areas including capacitor design and material behavior in electric fields.
Polarizability and Biological Molecules
Polarizability plays a significant role in biological systems. It affects the interactions between biomolecules, such as proteins and DNA, influencing their stability and function.
Factors Affecting Polarizability
Polarizability is influenced by several factors, including molecular shape, electron density distribution, and the presence of functional groups. These factors can either increase or decrease the overall polarizability of a molecule.
Measuring Polarizability
Scientists use various techniques to measure the polarizability of different molecules. These techniques include spectroscopic methods, computational simulations, and experimental observations.
Applications of Polarizability
Polarizability has numerous applications in fields such as materials science, drug development, and environmental chemistry. It provides insights into the behavior and properties of molecules, allowing for the design of new materials and the development of advanced technologies.
Conclusion
Understanding polarizability is crucial in the study of chemistry. It is a property that defines the extent to which a molecule can be distorted by an external electric field. In this article, we have explored ten fascinating facts about polarizability, shedding light on its significance in various chemical processes.
We’ve learned that polarizability plays a vital role in determining the strength of intermolecular forces, influencing the physical and chemical properties of substances. The ability of molecules to polarize affects their reactivity and solubility, making polarizability a key factor in understanding chemical reactions.
Additionally, we’ve discovered that polarizability is influenced by factors such as molecular size, shape, and electron distribution. It can vary significantly across different molecules, leading to diverse behaviors and interactions in chemical systems.
Overall, understanding polarizability helps us comprehend the intricate nature of chemical bonding, molecular interactions, and the properties of substances. Its study is essential in fields such as materials science, catalysis, and pharmaceutical research, paving the way for scientific advancements and innovation.
FAQs
Q: What is polarizability?
A: Polarizability refers to the ability of a molecule to be deformed or distorted by an external electric field.
Q: How is polarizability measured?
A: Polarizability is typically measured using spectroscopic techniques such as infrared and Raman spectroscopy.
Q: What factors affect polarizability?
A: Factors such as molecular size, shape, and electron distribution influence polarizability.
Q: How does polarizability affect intermolecular forces?
A: Polarizability determines the strength of intermolecular forces, which in turn influences physical and chemical properties of substances.
Q: What is the significance of polarizability in chemical reactions?
A: Polarizability affects the reactivity and solubility of molecules, influencing the course and outcome of chemical reactions.
Polarizability's captivating world offers endless fascination, but don't stop exploring now! Delving deeper into chemical bonding, the HSAB theory provides astonishing insights into how acids and bases interact. Uncover the mysteries of this powerful concept and gain a deeper understanding of chemistry's fundamental principles.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
