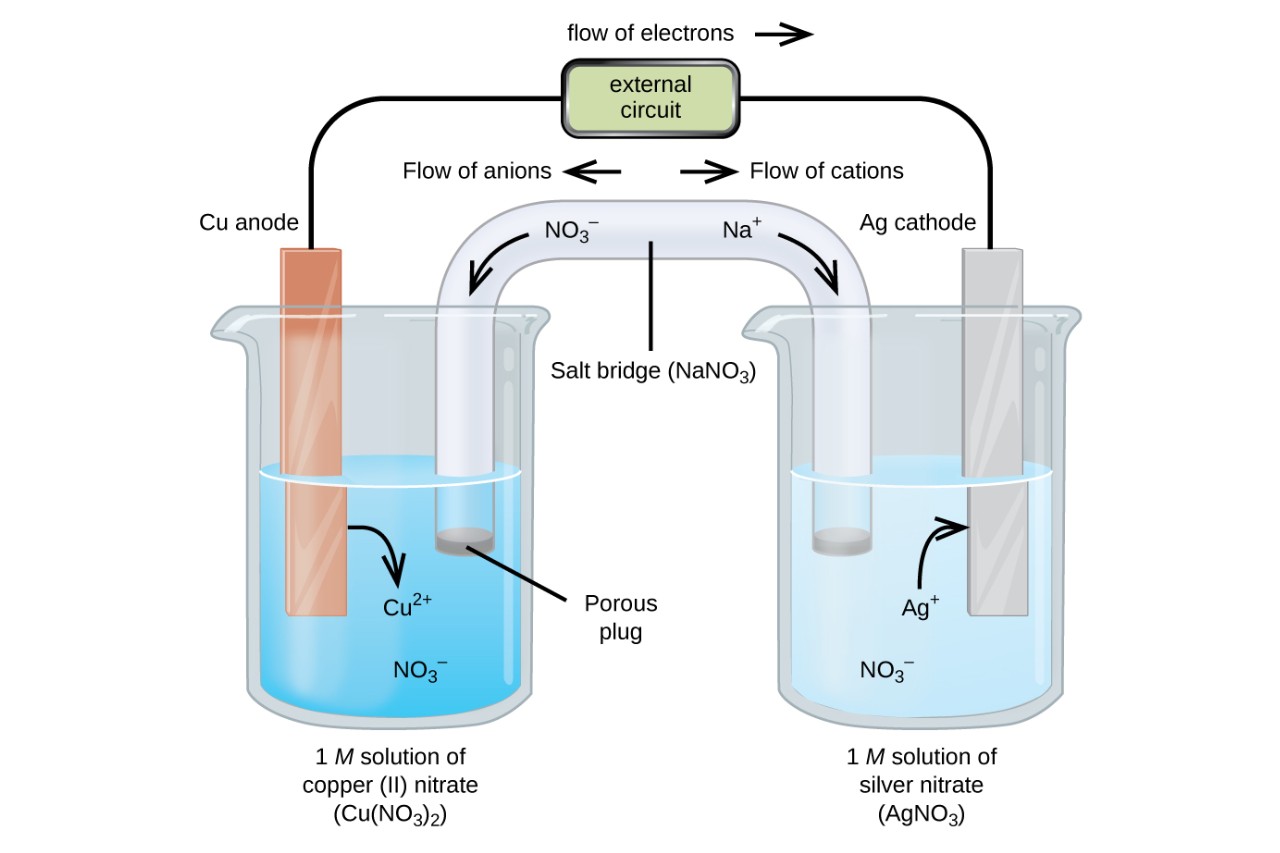
An electrochemical cell is a device that converts chemical energy into electrical energy through a series of redox reactions. This fascinating field of study combines the principles of chemistry and electricity to create devices that are essential to modern life.
In this article, we will explore 15 surprising facts about electrochemical cells that will not only expand your knowledge but also captivate your interest. From the invention of the first battery to the development of advanced fuel cells, the world of electrochemistry has revolutionized various industries and opened doors to new possibilities.
So, get ready to delve into the intriguing world of electrochemical cells and discover the science behind these remarkable devices. Whether you are a chemistry enthusiast or simply curious about how batteries power our everyday lives, these facts will surely provide you with a fresh perspective on the electrochemical wonders that surround us.
Key Takeaways:
- Electrochemical cells are like the hidden superheroes of our daily lives, powering everything from our smartphones to electric vehicles. They even help our bodies transmit signals through nerves, allowing us to move and think!
- These amazing cells are not just limited to batteries; they also enable sustainable energy solutions like solar cells and fuel cells, offering a greener future. Plus, they’re being explored for grid-scale energy storage and are crucial for powering electric vehicles.
Electrochemical cells are the heart of batteries
Did you know that electrochemical cells form the core of batteries? Batteries consist of one or more electrochemical cells connected in series or parallel to generate the desired voltage and current. This fundamental concept is the basis for the functionality of portable power sources that we heavily rely on.
Electrochemical cells can be found in nature
Electrochemical cell processes are not limited to man-made devices; they also occur naturally. For example, our body relies on electrochemical cells to transmit signals through nerves, allowing us to move, feel, and think. These cells, known as biological electrochemical cells, are responsible for our intricate neural network.
Electrochemical cells enable sustainable energy solutions
Renewable energy sources such as solar cells and fuel cells operate based on electrochemical principles. Solar cells convert sunlight into electrical energy, while fuel cells produce electricity from the reaction of hydrogen and oxygen. These clean and sustainable energy solutions hold great promise for a greener future.
The first electrochemical cell was invented by Alessandro Volta
In 1800, Italian physicist Alessandro Volta invented the first true electrochemical cell known as the Voltaic pile. This groundbreaking discovery laid the foundation for the development of modern batteries and marked the birth of electrochemistry as a scientific discipline.
Electrochemical cells come in diverse types
There are various types of electrochemical cells, each with its unique properties and applications. These include galvanic cells, electrolytic cells, fuel cells, and redox flow batteries. Each type serves a specific purpose, catering to different energy storage and conversion needs.
Electrochemical cells require a separation of reactants
In order for an electrochemical cell to function effectively, there must be a physical separation between the reactants. This is typically achieved using a porous membrane or an ion-conductive electrolyte, preventing direct contact between the reactants while allowing the flow of charged particles.
Electrochemical cells are reversible systems
One of the remarkable features of electrochemical cells is their ability to operate in both directions of electron flow, making them reversible systems. This means that a galvanic cell can be converted into an electrolytic cell and vice versa by changing the direction of electric current.
Electrochemical cells have their limitations
While electrochemical cells offer numerous advantages, they also have some limitations. These include self-discharge, limited lifespan, and the need for proper disposal due to environmental concerns. Research is ongoing to overcome these limitations and enhance the performance and sustainability of electrochemical cells.
Electrochemical cells power implantable medical devices
Implantable medical devices, such as pacemakers and insulin pumps, rely on electrochemical cells for their operation. These miniature power sources provide a reliable and long-lasting supply of energy, ensuring the uninterrupted functioning of life-saving medical devices.
Electrochemical cells enable electroplating
Through the process of electroplating, electrochemical cells are used to deposit a layer of metal onto the surface of an object. This technique is widely employed to enhance the appearance, protect against corrosion, or increase conductivity of materials.
The efficiency of electrochemical cells can be improved
Ongoing research aims to improve the efficiency of electrochemical cells by optimizing electrode materials, electrolytes, and cell design. Advancements in materials science and nanotechnology hold great potential for enhancing the energy storage and conversion capabilities of electrochemical cells.
Electrochemical cells play a role in environmental remediation
Electrochemical cells can be utilized for environmental purposes, such as water treatment and soil remediation. Electrochemical techniques such as electrocoagulation and electrokinetic remediation offer effective and sustainable solutions for addressing pollution and contamination issues.
Miniaturized electrochemical cells power wearable devices
Modern wearable devices, such as fitness trackers and smartwatches, rely on miniature electrochemical cells for their power supply. These compact and lightweight cells allow for longer battery life while ensuring a seamless user experience.
Electrochemical cells are being explored for grid-scale energy storage
As energy demands continue to rise, the development of efficient and cost-effective grid-scale energy storage solutions becomes essential. Electrochemical cells, such as redox flow batteries, are being extensively researched as a potential solution for storing excess renewable energy on a large scale.
Electrochemical cells power electric vehicles
The increasing popularity of electric vehicles is heavily reliant on electrochemical cells, specifically lithium-ion batteries. These high-performance batteries deliver the necessary power and range required for electric transportation, paving the way for a cleaner and more sustainable future.
From powering our everyday devices to driving sustainable energy solutions, electrochemical cells undoubtedly play a significant role in our lives. Understanding these 15 surprising facts about electrochemical cells not only broadens our knowledge but also highlights the incredible potential they hold for powering the world around us.
Conclusion
In conclusion, electrochemical cells are fascinating devices that play a crucial role in various aspects of our daily lives. Through the process of converting chemical energy into electrical energy, these cells power numerous electronic devices, from smartphones and laptops to cars and even spacecraft.
Throughout this article, we have uncovered some surprising facts about electrochemical cells. We have learned about the two main types of cells – galvanic cells and electrolytic cells – and how they function differently. We have also explored the concept of redox reactions and how they drive the generation of electrical energy.
Furthermore, we have delved into the applications and importance of electrochemical cells in areas such as renewable energy, medicine, and environmental conservation. From powering electric vehicles and storing energy from renewable sources to supporting medical implants and assisting in wastewater treatment, electrochemical cells have a significant impact on our lives.
As we continue to advance technologically and strive for a sustainable future, understanding and harnessing the power of electrochemical cells will undoubtedly play a vital role in shaping the world we live in.
FAQs
Q: What is an electrochemical cell?
A: An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions.
Q: What are the two main types of electrochemical cells?
A: The two main types of electrochemical cells are galvanic cells (also known as voltaic cells) and electrolytic cells.
Q: How do galvanic cells work?
A: Galvanic cells produce electrical energy through spontaneous redox reactions that occur between two different metals or metal-ion solutions.
Q: What is the purpose of an electrolytic cell?
A: Electrolytic cells are used to drive non-spontaneous redox reactions by applying an external electrical current.
Q: What are some applications of electrochemical cells?
A: Electrochemical cells have various applications, including powering electronic devices, energy storage, electroplating, and even medical implants.
Q: Can electrochemical cells be used in renewable energy?
A: Yes, electrochemical cells play a crucial role in renewable energy technologies such as solar cells, fuel cells, and batteries for storing energy from renewable sources.
Q: Are electrochemical cells environmentally friendly?
A: Electrochemical cells can be environmentally friendly, especially when used in renewable energy applications, as they produce clean energy with minimal greenhouse gas emissions.
Q: Can electrochemical cells be used in medical implants?
A: Yes, miniature electrochemical cells are often used in medical implants such as pacemakers and cochlear implants to provide a constant and reliable power source.
Q: Do electrochemical cells have any impact on environmental conservation?
A: Yes, electrochemical cells are used in wastewater treatment processes to remove pollutants and purify water, contributing to environmental conservation efforts.
Electrochemical cells hold countless surprises, from powering everyday devices to enabling groundbreaking technologies. Unraveling the Nernst equation's captivating facts sheds light on how these cells function at their core. Daniell cells, with their fascinating history and inner workings, showcase the ingenuity behind early electrochemical discoveries. Exploring these topics further will deepen your understanding of the principles governing electrochemical cells and their far-reaching applications. Stay curious and keep learning about the amazing world of electrochemistry!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
