Get ready to have your mind blown by these 19 fascinating facts about electrolysis! Electrolysis is a branch of chemistry that deals with the process of using an electric current to drive a chemical reaction. It has wide-ranging applications in various fields, including industry, medicine, and even everyday life. Understanding the principles and mechanisms behind electrolysis is not only intriguing but also essential for harnessing its potential. In this article, we will delve into the world of electrolysis and uncover some mind-blowing facts that will leave you truly amazed. From its historical roots to cutting-edge advancements, prepare to be electrified by the astonishing possibilities offered by this captivating chemical process.
Key Takeaways:
- Electrolysis is the only FDA-approved method for permanent hair removal, making it a safe and effective solution for unwanted hair on any part of the body. Say goodbye to constant shaving and embrace the long-lasting results of electrolysis!
- With minimal side effects and the ability to treat ingrown hairs and hormonal imbalances, electrolysis offers a virtually painless and customizable solution for individuals of all ages. It’s a time-tested, confidence-boosting investment in permanent hair removal.
Electrolysis is a method of permanent hair removal.
Electrolysis is the only FDA-approved method for permanent hair removal. It uses a small electric current to destroy the hair follicle, preventing regrowth.
Electrolysis can be used on any part of the body.
Whether it’s unwanted facial hair, underarm hair, or bikini line hair, electrolysis can safely and effectively remove hair from any part of the body.
The process of electrolysis works by inserting a small probe into the hair follicle.
During the electrolysis procedure, a certified technician inserts a tiny probe into each individual hair follicle. The probe then delivers a controlled electric current to destroy the follicle.
Electrolysis can be used on all hair colors and skin types.
Unlike other hair removal methods, electrolysis is suitable for all hair colors, including blonde, red, and gray. It is also safe for all skin types, including sensitive and tanned skin.
Electrolysis is more effective than other hair removal methods.
While methods like waxing and shaving offer temporary hair removal, electrolysis provides permanent results. It targets the hair follicle, preventing future hair growth.
Electrolysis is a safe and FDA-approved procedure.
Electrolysis has been used for decades and is considered a safe and effective method of hair removal. It is approved by the FDA as a permanent hair removal solution.
Electrolysis can help treat ingrown hairs.
Ingrown hairs can be painful and unsightly. Electrolysis can effectively treat and eliminate ingrown hairs, preventing them from reoccurring.
Multiple sessions of electrolysis are required for permanent results.
Since hair grows in cycles, multiple sessions of electrolysis are necessary to target all the hair follicles. Typically, several sessions are needed to achieve permanent hair removal.
Electrolysis is virtually painless.
Modern electrolysis techniques, such as the use of topical numbing creams, have made the procedure virtually pain-free. Most people only experience a slight tingling sensation during the treatment.
Electrolysis can be customized to each individual’s needs.
Every person’s hair growth pattern is unique. Electrolysis allows the technician to customize the treatment to target specific areas and adjust the intensity of the electric current accordingly.
Electrolysis can help with hormonal imbalances.
Excessive hair growth due to hormonal imbalances, such as polycystic ovary syndrome (PCOS), can be effectively treated with electrolysis. It provides a long-term solution for managing unwanted hair growth.
Electrolysis is a time-tested method.
Electrolysis has been used for over 100 years and has a proven track record of delivering permanent hair removal results. It is a trusted and reliable solution for those seeking long-term hair removal.
Electrolysis can boost self-confidence.
Unwanted hair can have a negative impact on self-esteem. Electrolysis offers a permanent solution, allowing individuals to feel more confident and comfortable in their own skin.
Electrolysis has minimal side effects.
Common side effects of electrolysis, such as redness and swelling, are temporary and subside quickly. When performed by a trained professional, electrolysis is a low-risk procedure.
Electrolysis is suitable for all ages.
Whether you’re a teenager experiencing unwanted hair growth or an adult looking for a permanent hair removal solution, electrolysis is safe and effective for all age groups.
Electrolysis is a long-lasting investment.
While electrolysis may require multiple sessions, once the hair follicles are permanently destroyed, no further treatment is necessary. It is a worthwhile investment for those seeking a permanent hair removal solution.
Electrolysis can be combined with other hair removal methods.
For some individuals, combining electrolysis with other hair removal methods, such as laser hair removal or waxing, can provide enhanced results and target different hair growth stages.
Electrolysis can be performed by certified professionals.
To ensure safe and effective electrolysis, it is essential to seek the services of a certified electrologist. They have undergone extensive training and will follow strict hygiene and safety protocols.
Electrolysis is a permanent solution.
Unlike temporary hair removal methods, electrolysis provides a permanent solution for unwanted hair growth. Once the hair follicles are destroyed, hair will not grow back.
These 19 mind-blowing facts about electrolysis reaffirm its position as the ultimate solution for permanent hair removal. Whether you’re battling unwanted facial hair or seeking freedom from constant shaving, electrolysis offers a safe, effective, and long-lasting hair removal method. Don’t let unwanted hair hold you back! Take advantage of the remarkable benefits of electrolysis and embrace the confidence and freedom it provides.
Conclusion
In conclusion, electrolysis is a fascinating process that has numerous applications and benefits. From removing unwanted hair to powering chemical reactions, electrolysis plays a significant role in various industries. Its ability to selectively break down compounds and produce desired products makes it an essential tool in chemistry and beyond.Through this article, we have explored 19 mind-blowing facts about electrolysis. We learned about the principles behind electrolysis, the different types of electrolysis cells, and the key factors that influence its efficiency. We also discovered how electrolysis is used in various fields, including energy storage, metal extraction, and water treatment.Electrolysis continues to advance, opening new possibilities in fields such as fuel cells, renewable energy, and materials science. As technology improves, electrolysis is poised to play an even more significant role in shaping our future.So next time you encounter the word “electrolysis,” remember the incredible power behind it and the wide range of applications it offers. It truly is a phenomenon that showcases the wonders of chemistry.
FAQs
1. What is electrolysis?
Electrolysis is a chemical process that uses an electric current to drive a non-spontaneous chemical reaction, breaking down a compound into its constituent elements or producing desired products.
2. How does electrolysis work?
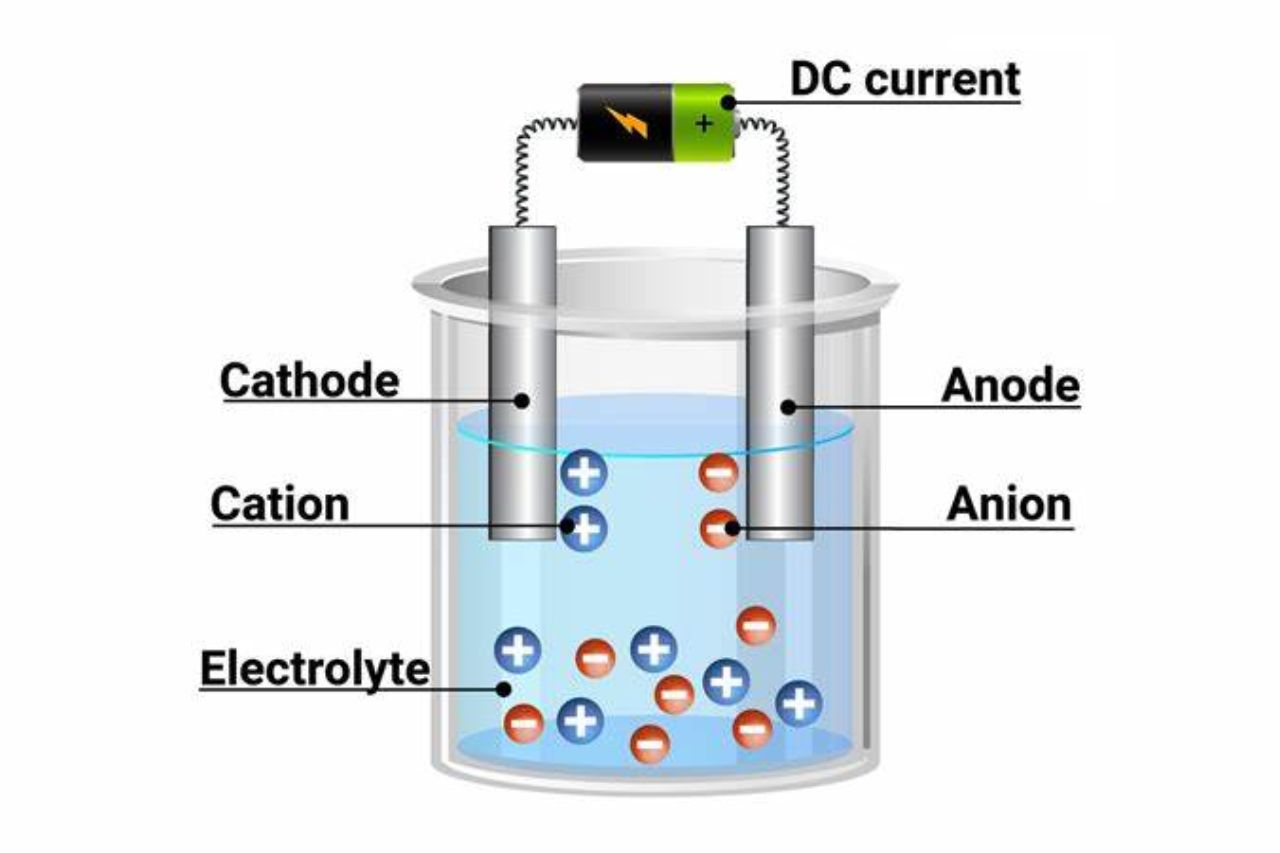
During electrolysis, an electric current is passed through an electrolyte solution or a molten compound. The current causes the positive ions (cations) to move towards the negative electrode (cathode) and the negative ions (anions) to move towards the positive electrode (anode).
3. What are some applications of electrolysis?
Electrolysis has a wide range of applications, including industrial metal extraction, water treatment, production of chemicals and fuels, and even hair removal.
4. What are the different types of electrolysis cells?
There are various types of electrolysis cells, such as the basic electrolytic cell, the membrane cell, the diaphragm cell, and the solid oxide electrolysis cell (SOEC).
5. Is electrolysis an efficient process?
The efficiency of electrolysis depends on several factors, including the electrical energy input, the electrode materials, and the nature of the electrolyte. Researchers are continually working on improving the efficiency to make electrolysis more sustainable.
6. Can electrolysis be used for energy storage?
Yes, electrolysis can be used for energy storage by converting excess electrical energy into chemical potential energy in the form of hydrogen gas, which can later be reconverted into electricity using fuel cells.
7. Are there any environmental benefits of electrolysis?
Yes, electrolysis can contribute to sustainability by enabling the production of green hydrogen, which is a clean fuel that can be used as an alternative to fossil fuels.
8. Is electrolysis a safe process?
Electrolysis can be safe if appropriate precautions are taken. Handling electricity and chemicals always require careful handling and adherence to safety protocols.
9. Can electrolysis be used for water treatment?
Yes, electrolysis can be used for water treatment by removing contaminants, disinfecting water, and producing chemicals such as chlorine for disinfection purposes.
10. Are there any future developments in electrolysis?
Yes, researchers are actively exploring new materials and techniques to make electrolysis more efficient, cost-effective, and applicable in various emerging fields such as energy conversion and materials synthesis.
Electrolysis is a fascinating process with numerous applications, from hair removal to industrial processes. If you're curious to learn more about this intriguing topic, consider exploring the laws of electrolysis discovered by Michael Faraday, which laid the foundation for modern electrochemistry. Faraday's groundbreaking work revolutionized our understanding of the relationship between electricity and chemical reactions, paving the way for countless innovations in science and technology. So why not satisfy your curiosity and delve into the captivating world of electrolysis and electrochemistry? You might just be surprised by the extraordinary facts waiting to be uncovered.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
