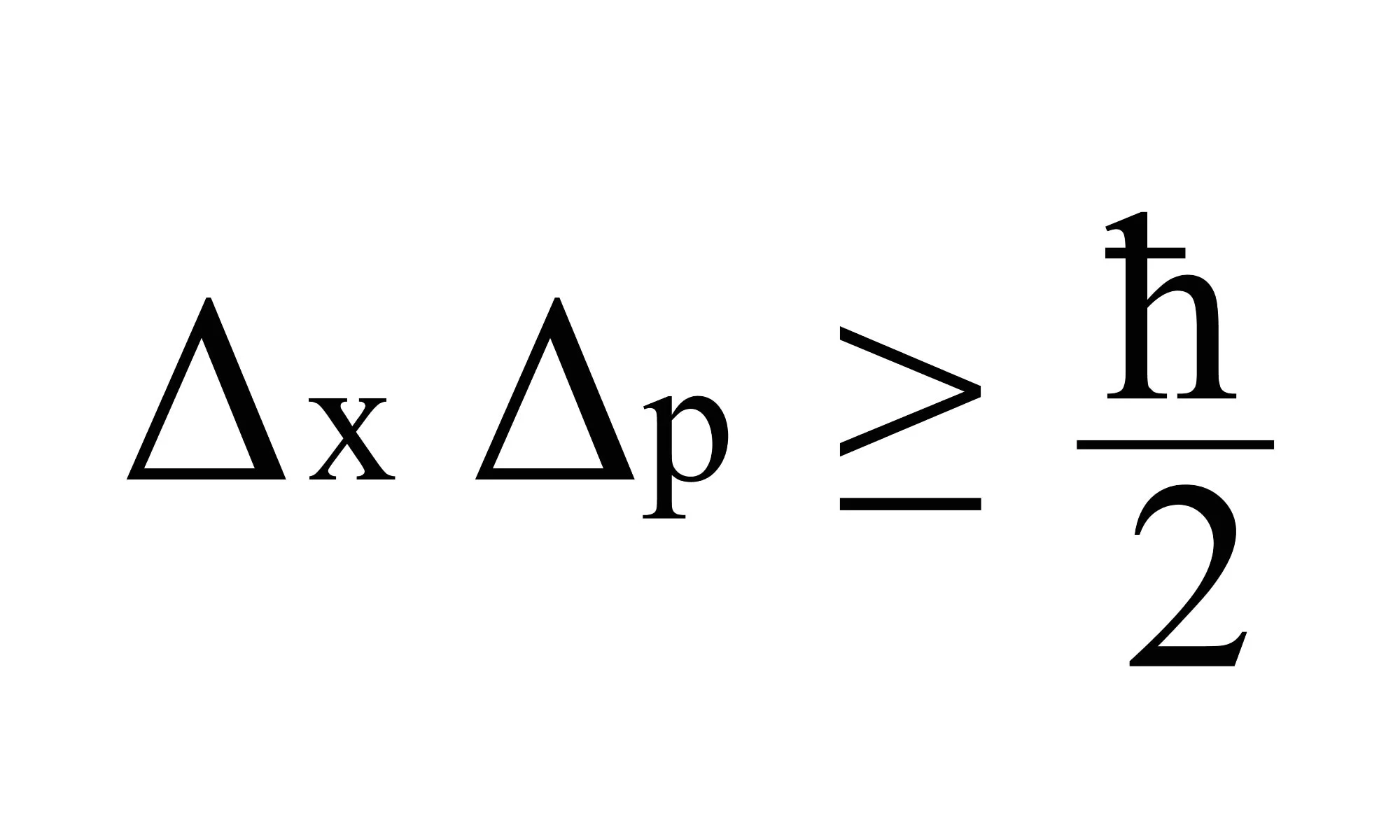
The Uncertainty Principle is a fundamental concept in the field of quantum mechanics that revolutionized our understanding of the universe. Proposed by German physicist Werner Heisenberg in 1927, this principle challenges our conventional notions of determinism and causality. It states that there is a fundamental limit to the precision with which certain pairs of physical properties, such as position and momentum, can be simultaneously known. In other words, the more accurately we measure one of these properties, the less accurately we can simultaneously measure the other.
While the Uncertainty Principle may seem counterintuitive, it has profound implications for our understanding of the microscopic world. It has sparked numerous debates, experiments, and philosophical discussions among physicists and philosophers alike. In this article, we will explore 17 fascinating facts about the Uncertainty Principle that will shed light on its significance and intricacies.
Key Takeaways:
- The Uncertainty Principle, introduced by Werner Heisenberg, shows that we can’t know everything about a particle at the same time. This challenges our idea of a predictable universe and has big implications for quantum mechanics.
- The Uncertainty Principle isn’t just a fancy theory—it’s been proven through experiments! It also affects things like quantum computing and even the concept of reality itself. It’s a mind-bending concept that keeps scientists curious and excited.
The Uncertainty Principle was introduced by Werner Heisenberg in 1927.
The Uncertainty Principle, also known as Heisenberg’s Uncertainty Principle, was formulated by German physicist Werner Heisenberg in 1927 as a fundamental concept in quantum mechanics.
It states that there is a limit to how precisely certain pairs of physical properties of a particle can be known simultaneously.
The Uncertainty Principle asserts that, for certain pairs of physical properties, such as position and momentum, the more precisely one property is known, the less precisely the other can be known. This fundamental limitation arises due to the wave-particle duality of quantum mechanics.
The Uncertainty Principle challenges the classical notion of determinism.
Unlike classical physics, which suggests that the properties of particles can be known with absolute certainty, the Uncertainty Principle introduces the concept of inherent uncertainty and unpredictability at the quantum level.
It applies to all particles, not just subatomic particles.
The Uncertainty Principle is applicable to all particles, regardless of their size or nature. It is not limited to just subatomic particles like electrons or photons.
It has profound implications for the measurement and observation of quantum systems.
The Uncertainty Principle raises key questions about the nature of measurement in the quantum world. It suggests that the act of observation itself can influence and alter the properties of particles, making precise simultaneous measurement impossible.
The Uncertainty Principle is closely related to wave-particle duality.
Wave-particle duality is a fundamental concept in quantum mechanics that suggests that particles can exhibit both wave-like and particle-like properties. The Uncertainty Principle arises due to this inherent duality, where the precise position and momentum of a particle cannot be known simultaneously.
It paved the way for the development of quantum mechanics.
Heisenberg’s formulation of the Uncertainty Principle was a crucial step in the development of quantum mechanics as a new framework for understanding the behavior of particles at the microscopic level.
The Uncertainty Principle is not a result of measurement limitations.
Contrary to popular misconceptions, the Uncertainty Principle is not caused by technological or measurement limitations. It is an intrinsic property of the quantum world itself.
The Uncertainty Principle has connections to the concept of energy-time uncertainty.
In addition to position and momentum, the Uncertainty Principle can also be applied to other physical quantities, such as energy and time. This energy-time uncertainty relationship has significant implications in various areas of physics.
The Uncertainty Principle challenges the notion of a completely deterministic universe.
By introducing inherent uncertainty into the fabric of reality, the Uncertainty Principle challenges the classical notion of a completely deterministic universe, where all events can be predicted with absolute certainty.
It has been experimentally confirmed through various scientific observations.
The Uncertainty Principle has been validated through numerous experiments and observations in the field of quantum mechanics. These experiments provide empirical evidence for the limitations on the simultaneous knowledge of certain particle properties.
It has implications for the quantum computing and cryptography fields.
The Uncertainty Principle has practical implications beyond fundamental physics. In the fields of quantum computing and cryptography, the principle has implications for the limitations and possibilities of information processing and encryption.
The Uncertainty Principle has connections to the concept of quantum entanglement.
Quantum entanglement is a phenomenon in which particles become correlated in such a way that the state of one particle is dependent on the state of another, regardless of the distance between them. The Uncertainty Principle is related to the limitations on simultaneous knowledge of entangled particles.
The Uncertainty Principle allows for the existence of virtual particles.
Virtual particles are particles that briefly pop in and out of existence. The Uncertainty Principle permits these fluctuations in the quantum vacuum, where particles and antiparticles spontaneously arise and annihilate each other in a short span of time.
The Uncertainty Principle has implications for the concept of objective reality.
Due to the Uncertainty Principle, the concept of a fixed and objective reality becomes muddled in the realm of quantum mechanics. It suggests that reality is inherently probabilistic and depends on the act of observation.
The Uncertainty Principle is a subject of ongoing research and debate.
Despite decades of study, the interpretation and implications of the Uncertainty Principle continue to be explored and debated by physicists. The principle remains a fascinating and active area of research in the field of quantum mechanics.
The Uncertainty Principle challenges our intuitive understanding of the physical world.
By introducing fundamental uncertainty into the fabric of reality, the Uncertainty Principle challenges our everyday intuitions about how the physical world behaves. It underscores the bizarre and counterintuitive nature of the quantum realm.
In conclusion, the 17 Fascinating Facts About Uncertainty Principle shed light on the profound implications of this fundamental concept in quantum mechanics. From challenging determinism to influencing the fields of quantum computing and cryptography, the Uncertainty Principle continues to captivate and intrigue scientists as they explore the mysteries of the quantum world.
Conclusion
In conclusion, the uncertainty principle is a fundamental concept in quantum physics that has revolutionized our understanding of the subatomic world. It states that there is a limit to the precision with which certain pairs of physical properties, such as position and momentum, can be known simultaneously. This principle has far-reaching implications, influencing fields ranging from particle physics to the study of black holes.
While the uncertainty principle may seem counterintuitive, it highlights the inherent nature of quantum mechanics, where particles can exist in multiple states simultaneously and their properties are described in terms of probabilities. It challenges our classical understanding of determinism and calls for a probabilistic view of the universe.
Exploring the uncertainty principle further can lead to a deeper understanding of the nature of reality and the limits of human knowledge. It continues to be an area of active research and has inspired groundbreaking discoveries in the field of quantum physics.
FAQs
1. What is the uncertainty principle?
The uncertainty principle is a principle in quantum physics that states that there is a limit to the precision with which certain pairs of physical properties can be known simultaneously. It implies that the more accurately we measure one property, such as position, the less accurately we can know another, such as momentum.
2. Who formulated the uncertainty principle?
The uncertainty principle was formulated by the German physicist Werner Heisenberg in 1927. Heisenberg’s groundbreaking work revolutionized the field of quantum mechanics and earned him the Nobel Prize in Physics in 1932.
3. What are the implications of the uncertainty principle?
The uncertainty principle has profound implications for our understanding of the subatomic world. It challenges the classical notion of determinism and introduces a probabilistic view of reality. It also poses limits on the precision with which we can measure certain properties, which has practical implications in fields like nanotechnology and atomic spectroscopy.
4. Can the uncertainty principle be overcome?
No, the uncertainty principle is a fundamental principle of quantum mechanics. It is inherent in the nature of particles at the quantum level. While there are techniques to minimize the uncertainties in certain measurements, they cannot be completely eliminated due to the inherent probabilistic nature of quantum systems.
5. How does the uncertainty principle relate to everyday life?
Although the uncertainty principle is a fundamental concept in quantum physics, its effects are not typically observable in everyday life. At the macroscopic scale, objects obey the laws of classical physics, which are deterministic and predictable. The uncertainty principle becomes significant only when dealing with particles at the subatomic level.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
