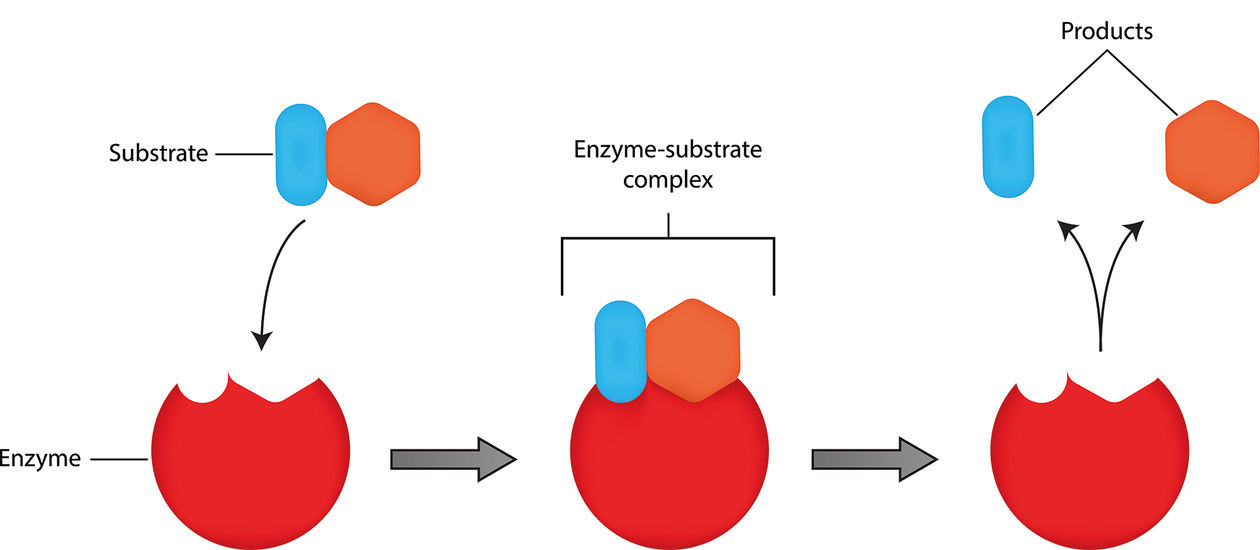
Enzymes are fascinating biological molecules that play a crucial role in almost every chemical reaction that takes place in our bodies. One of the fundamental concepts in enzymology is the enzyme-substrate complex, which occurs when an enzyme and a substrate come together to initiate a chemical reaction. The enzyme-substrate complex is essential for the efficient functioning of enzymes and is a topic that continues to captivate researchers and scientists alike.
In this article, we will delve into the world of enzymes and explore nine mind-blowing facts about the enzyme-substrate complex. From the intricacies of enzyme specificity and catalytic efficiency to the remarkable ways in which enzymes enhance chemical reactions, we will uncover the hidden secrets of this remarkable interaction. So, get ready to be amazed as we unravel the mysteries of the enzyme-substrate complex and discover why it is truly a marvel of biochemical science.
Key Takeaways:
- Enzyme-substrate complex is like a special handshake between enzymes and substrates, making chemical reactions happen faster in living things.
- Enzymes are picky about their substrates, and their “hand-in-glove” fit allows for efficient and specific reactions in the body.
The enzyme-substrate complex is key to catalysis.
The enzyme-substrate complex plays a crucial role in catalyzing chemical reactions in living systems. Enzymes, as biological catalysts, bind to specific substrates to form this complex, enabling the reaction to occur at a faster rate.
Enzymes are highly specific in recognizing their substrates.
Enzymes exhibit a remarkable level of specificity towards their substrates. Through a lock-and-key or induced fit mechanism, enzymes only bind to specific substrates that complement their active sites, ensuring efficient catalysis.
The enzyme-substrate complex undergoes conformational changes.
When a substrate binds to an enzyme, it induces conformational changes in both the enzyme and the substrate. This conformational change facilitates the formation of the enzyme-substrate complex and allows the reaction to proceed.
Enzyme-substrate interaction involves multiple weak interactions.
The formation of the enzyme-substrate complex is governed by various weak interactions such as hydrogen bonding, electrostatic interactions, and van der Waals forces. These interactions stabilize the complex and enable efficient catalysis.
Enzymes can alter the reaction rate by adjusting the affinity for the substrate.
Enzymes can modulate the rate of a reaction by adjusting their affinity for the substrate. This can be achieved through factors such as pH, temperature, and the presence of inhibitors or activators.
The enzyme-substrate complex is transient.
The enzyme-substrate complex is not a permanent structure but rather a transient intermediate in the catalytic cycle. Once the reaction is complete, the enzyme releases the products and is available to bind with other substrates.
Enzymes can undergo cooperative binding.
In certain cases, enzymes can exhibit cooperative binding behavior, where binding of one substrate molecule enhances the enzyme’s affinity for subsequent substrate molecules. This allows for efficient utilization of substrates and regulation of enzymatic activity.
The rate of enzyme-substrate complex formation can be influenced by factors like substrate concentration.
The rate at which the enzyme-substrate complex is formed is dependent on the concentration of the substrate. At lower substrate concentrations, the rate of complex formation may be limited, leading to slower reaction rates.
Enzyme-substrate complexes can be reversible.
In some cases, the binding between an enzyme and substrate can be reversible. This means that the complex can dissociate back into the enzyme and substrate. This reversibility allows for dynamic regulation of enzymatic activity.
In conclusion, the enzyme-substrate complex is a fascinating interaction that drives biochemical reactions in living organisms. Its specificity, conformational changes, and transient nature contribute to the efficiency of enzymatic catalysis. Understanding the intricacies of the enzyme-substrate complex is crucial in unraveling the complex world of biochemistry.
Conclusion
In conclusion, the enzyme-substrate complex is a fascinating concept in biochemistry that plays a crucial role in various biological processes. Understanding the intricacies of this molecular interaction can provide valuable insights into how enzymes function and catalyze chemical reactions.Throughout this article, we have explored nine mind-blowing facts about the enzyme-substrate complex. From the lock-and-key model to induced fit, from the significance of enzyme specificity to the effect of temperature and pH, these facts highlight the complexity and versatility of this biochemical phenomenon.The enzyme-substrate complex exhibits remarkable efficiency and specificity, enabling enzymes to carry out a wide range of biochemical reactions with precision. This crucial step in enzyme-catalyzed reactions sets the stage for the conversion of substrates into products, facilitating essential biological processes in our bodies.By delving into the world of enzyme-substrate complexes, we gain a deeper appreciation for the remarkable biochemical machinery that drives life. The more we understand these molecular interactions, the closer we come to unraveling the mysteries of the chemical reactions that sustain living organisms.
FAQs
Q: What is an enzyme-substrate complex?
A: An enzyme-substrate complex refers to the temporary association between an enzyme and its substrate during a biochemical reaction. This complex facilitates the conversion of substrate molecules into product molecules.
Q: How does the enzyme-substrate complex work?
A: The enzyme-substrate complex works by the specific binding of the enzyme to its substrate. This binding allows the enzyme to catalyze the chemical reaction by lowering the activation energy required, resulting in the conversion of the substrate into products.
Q: What is the importance of enzyme specificity in the formation of an enzyme-substrate complex?
A: Enzyme specificity is crucial in forming the enzyme-substrate complex. The enzyme’s active site is uniquely designed to complement the specific shape and chemical properties of its substrate. This ensures that only the correct substrate can bind to the enzyme, leading to efficient catalysis.
Q: How does temperature and pH affect the formation of the enzyme-substrate complex?
A: Temperature and pH can influence the formation of the enzyme-substrate complex. Extreme temperatures or pH levels can disrupt the interactions between the enzyme and substrate, affecting the efficiency of the complex formation and the catalytic activity of the enzyme.
Q: Can the enzyme-substrate complex be reversible?
A: Yes, the enzyme-substrate complex can be reversible. Once the reaction is completed, the products can dissociate from the enzyme, allowing the enzyme to bind to new substrate molecules and initiate another round of catalysis.
Q: What happens if the enzyme-substrate complex is disrupted?
A: If the enzyme-substrate complex is disrupted, the reaction may not proceed efficiently or may not occur at all. Disruption can be caused by factors such as denaturation of the enzyme or changes in the substrate or environmental conditions.
Q: Are all enzymes-substrate interactions specific?
A: Yes, enzymes-substrate interactions are specific. Each enzyme is designed to bind to a specific substrate or group of substrates. This specificity ensures that the enzyme only catalyzes the desired reaction in a precise and controlled manner.
Q: Can enzyme-substrate complexes be inhibited?
A: Yes, enzyme-substrate complexes can be inhibited. Inhibitors can bind to either the enzyme or the substrate, preventing their interaction and inhibiting the catalytic activity of the enzyme. This inhibition can be temporary or irreversible.
Q: Are enzyme-substrate complexes involved in everyday life?
A: Yes, enzyme-substrate complexes play a vital role in everyday life. They are involved in various biological processes, such as digestion, cellular respiration, DNA replication, and hormone regulation. Without enzyme-substrate complexes, these essential processes would not occur efficiently.
Enzyme-substrate complexes are truly mind-blowing! Their intricate dance of recognition, conformational changes, and catalytic prowess is nothing short of awe-inspiring. If you found these facts captivating, just wait until you explore the fascinating world of MichaelisMenten kinetics. Prepare to be amazed as you unravel the mysteries behind enzyme kinetics and how they shape the very fabric of life itself!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
