Alkanes are a versatile and intriguing class of organic compounds that play a significant role in various fields, from chemistry to everyday life. These hydrocarbons are composed solely of carbon and hydrogen atoms, making them a fundamental building block in organic chemistry. With their unique properties and wide-ranging applications, alkane compounds have captivated the attention of scientists and researchers for many years.
In this article, we will delve into the fascinating world of alkanes and uncover nine intriguing facts about these compounds. From their diverse structure to their importance in energy production and environmental impact, we will explore the many facets of alkane compounds. So, get ready to discover some exciting and mind-boggling information about these hydrocarbons that are all around us!
Key Takeaways:
- Alkanes are simple hydrocarbons found in natural sources, used as fuels, and play a crucial role in everyday life, from heating homes to powering vehicles.
- Alkanes serve as the building blocks for more complex organic compounds and play a crucial role in the Earth’s carbon cycle, impacting global climate change.
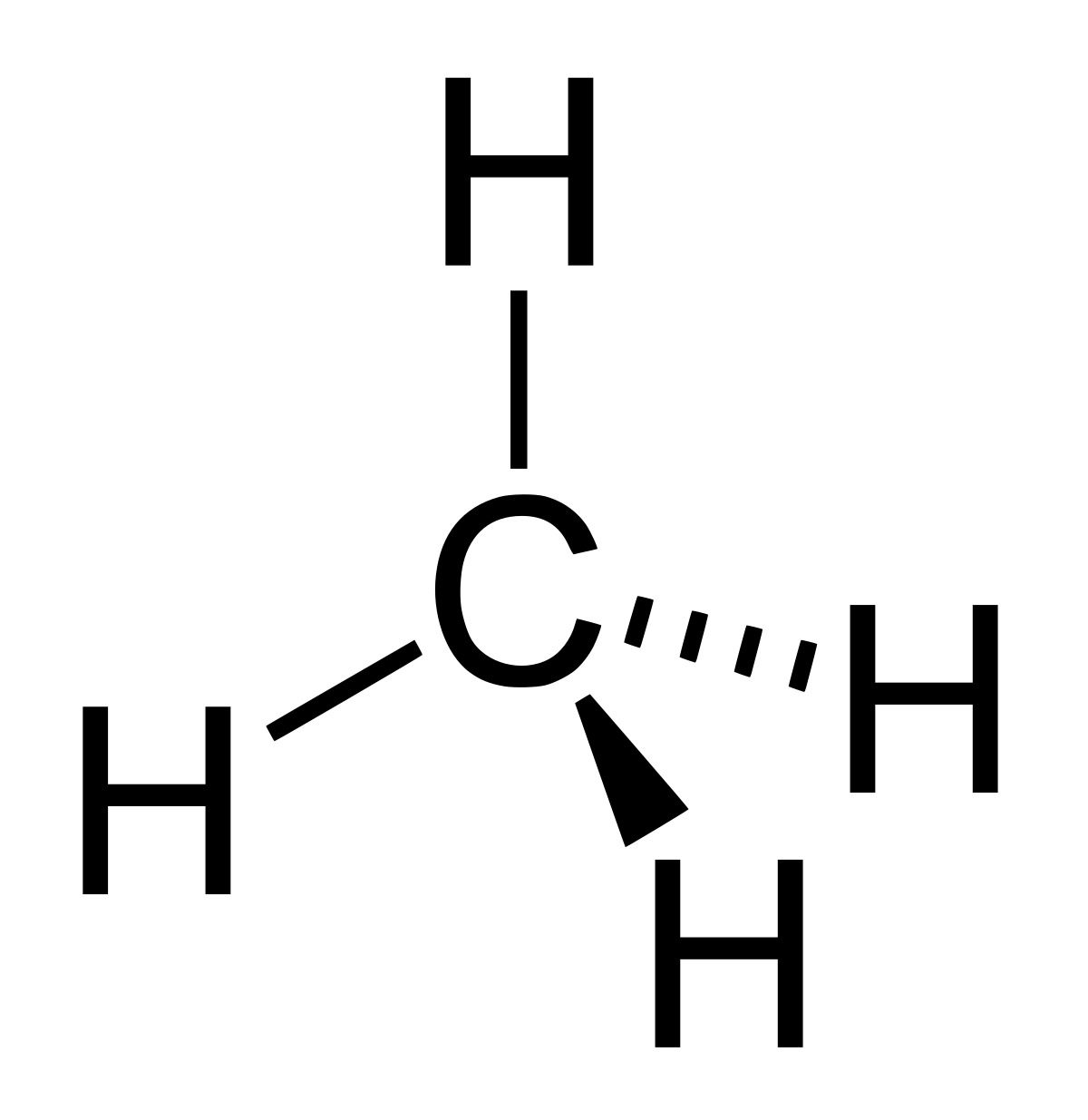
Alkanes are the simplest type of hydrocarbons.
Alkanes are organic compounds made up entirely of hydrogen and carbon atoms. They are known as “saturated” hydrocarbons because they contain only single bonds between carbon atoms.
Alkanes can be found in various natural sources.
Alkanes can be found in natural gas, petroleum, and even in some plants and animals. They are widely used as fuels due to their energy-rich nature.
Alkanes have multiple uses in everyday life.
From heating homes to powering vehicles, alkanes play a crucial role in our daily activities. They are also used as raw materials in the production of plastics, solvents, and lubricants.
Alkanes have a unique naming system.
Alkanes follow a systematic naming system based on the number of carbon atoms in the molecule. The names end with the suffix “-ane” and have prefixes to indicate the number of carbon atoms present.
Alkanes have diverse physical properties.
Alkanes vary in their physical properties depending on the number of carbon atoms in the molecule. They can range from being gaseous, like methane, to being solid at room temperature, like paraffin wax.
Alkanes can undergo combustion reactions.
When alkanes react with oxygen, they undergo combustion reactions, releasing large amounts of energy in the form of heat and light. This is why they are commonly used as fuels.
Alkanes can undergo various types of chemical reactions.
Alkanes can react with halogens, such as chlorine or bromine, to form haloalkanes. They can also undergo reactions like halogenation, oxidation, and cracking, leading to the formation of different compounds.
Alkanes serve as the building blocks for more complex organic compounds.
Alkanes are often used as starting materials in organic synthesis to create more complex compounds. They can be modified through chemical reactions to form alkenes, alcohols, and other functional groups.
Alkanes play a crucial role in the Earth’s carbon cycle.
Through natural processes like decomposition and fossil fuel formation, alkanes contribute to the Earth’s carbon cycle. Understanding their behavior and impact is essential for studying global climate change.
Conclusion
In summary, alkane compounds are truly fascinating in their properties and applications. From their simple structure to their important role in various industries, alkane compounds have contributed significantly to the world of chemistry. Their unique bonding and stability make them the building blocks for many organic compounds, allowing for the synthesis of countless useful materials.Understanding alkane compounds and their properties is essential in fields such as pharmaceuticals, fuels, and materials science. The ability to manipulate their structure and reactivity opens up new avenues for innovation and discovery. Whether it’s exploring the potential of new fuels or developing new drugs, alkane compounds continue to captivate scientists and researchers alike.By delving into the world of alkane compounds, we gain a deeper understanding of the fundamental principles of organic chemistry. This knowledge not only expands our scientific understanding but also paves the way for breakthroughs that can shape the future. So next time you come across an alkane compound, remember the intriguing world of possibilities it represents.
FAQs
1. What are some common examples of alkane compounds?
Some common examples of alkane compounds include methane, ethane, propane, and butane. These compounds are commonly found in natural gas and petroleum.
2. How are alkane compounds used in everyday life?
Alkane compounds are used as fuels for heating and cooking. They are also used as raw materials in the production of plastics, solvents, and other industrial chemicals.
3. Are all alkane compounds odorless?
No, not all alkane compounds are odorless. For example, methane, which is the main component of natural gas, has a characteristic odor that can be detected by smell.
4. Can alkane compounds be toxic?
While most alkane compounds are generally considered to be safe, some longer-chain alkane compounds can be toxic if ingested or inhaled in large quantities. It’s important to handle and use these compounds in a controlled and safe manner.
5. Can alkane compounds undergo chemical reactions?
Alkane compounds are generally known for their low reactivity due to their stable carbon-carbon and carbon-hydrogen bonds. However, under certain conditions, such as high temperature and pressure, alkane compounds can undergo reactions, such as combustion or halogenation.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
