Lead tetrafluoride might sound like a mouthful, but it's a fascinating compound with some surprising facts. What is lead tetrafluoride? Lead tetrafluoride is a chemical compound made up of lead and fluorine, with the formula PbF₄. This compound is known for being quite reactive and is used in various chemical processes. It's not something you'd find in your kitchen, but it plays a crucial role in the world of chemistry. From its unique properties to its applications, lead tetrafluoride holds many secrets. Ready to dive into the world of this intriguing compound? Let's explore 40 facts about lead tetrafluoride that will blow your mind!
Key Takeaways:
- Lead Tetrafluoride is a toxic compound used in research and material science. It has unique properties and requires strict safety measures for handling and disposal.
- Despite its toxicity, Lead Tetrafluoride has potential for new applications and ongoing research to minimize its environmental impact. Safety, innovation, and education are key for its future.
What is Lead Tetrafluoride?
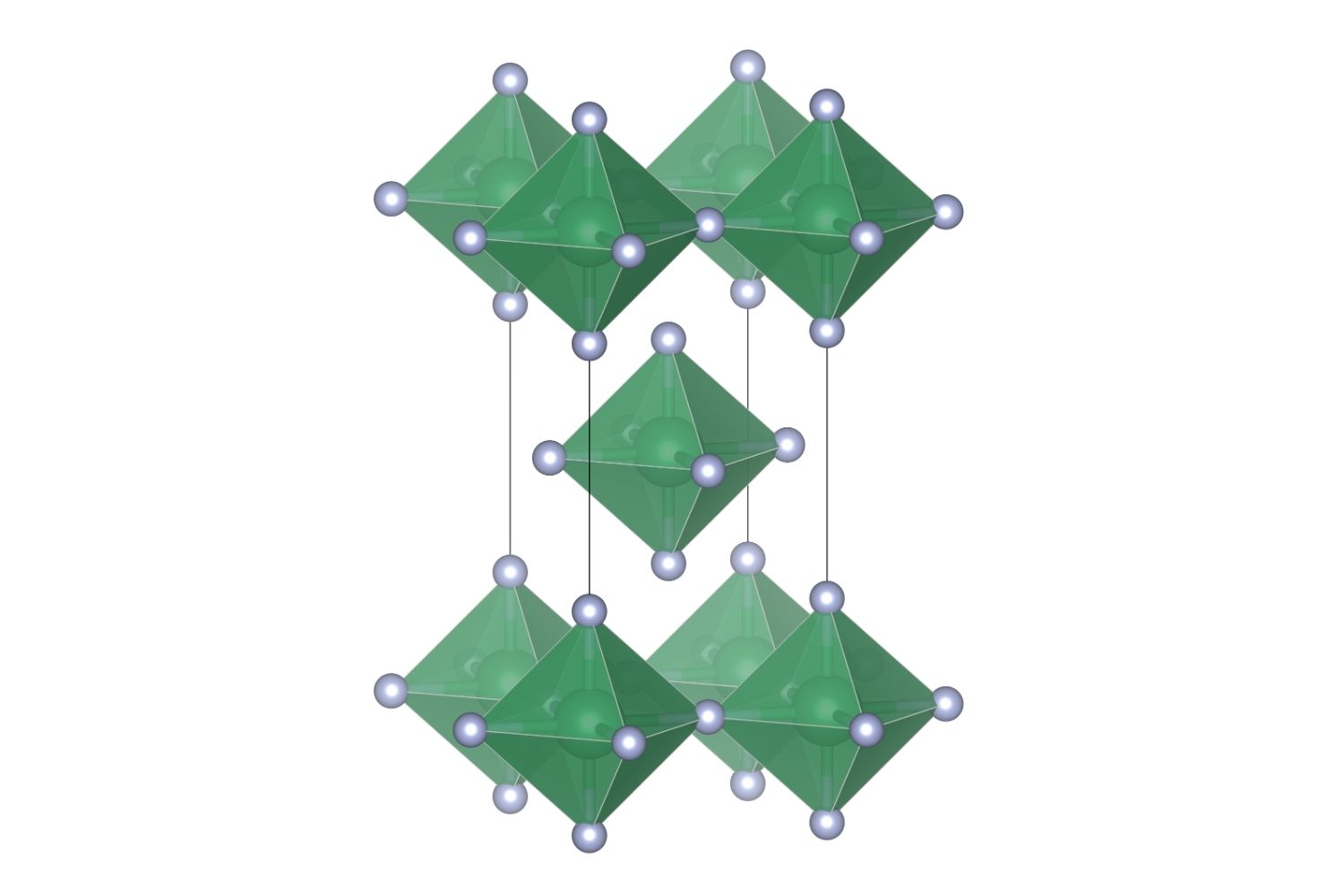
Lead tetrafluoride (PbF₄) is a chemical compound that combines lead and fluorine. It’s not something you encounter daily, but it has some fascinating properties and uses. Let’s dive into some intriguing facts about this unique substance.
-
Lead tetrafluoride is a white crystalline solid. It looks like tiny white crystals under a microscope.
-
It has the chemical formula PbF₄. This means each molecule contains one lead atom and four fluorine atoms.
-
PbF₄ is highly toxic. Handling it requires extreme caution due to its poisonous nature.
-
It’s not naturally occurring. Scientists create it in laboratories through chemical reactions.
-
Lead tetrafluoride is used in research. It helps scientists understand more about fluorine chemistry.
Properties of Lead Tetrafluoride
Understanding the properties of PbF₄ can help us grasp why it’s so special. Here are some key characteristics.
-
PbF₄ has a high melting point. It melts at around 600°C (1112°F).
-
It’s insoluble in water. This means it doesn’t dissolve when mixed with water.
-
The compound is stable at room temperature. It doesn’t break down or react easily under normal conditions.
-
PbF₄ is a strong oxidizing agent. It can cause other substances to lose electrons during chemical reactions.
-
It has a tetragonal crystal structure. This means its crystals form in a specific geometric pattern.
How is Lead Tetrafluoride Made?
Creating PbF₄ involves specific chemical processes. Here’s a look at how scientists produce it.
-
PbF₄ is made by reacting lead dioxide (PbO₂) with fluorine gas (F₂). This reaction takes place at high temperatures.
-
The process requires special equipment. Handling fluorine gas is dangerous, so scientists use specialized tools.
-
Safety measures are crucial. Protective gear and proper ventilation are essential when making PbF₄.
-
The reaction produces pure PbF₄ crystals. These are then collected and stored for use.
-
Purity is important. Impurities can affect the properties and behavior of PbF₄.
Uses of Lead Tetrafluoride
PbF₄ isn’t just a lab curiosity. It has practical applications, especially in scientific research.
-
It’s used in fluorine chemistry studies. Researchers study its reactions to learn more about fluorine.
-
PbF₄ helps in material science. Scientists explore its properties to develop new materials.
-
It’s a reference material. Researchers use it to compare and calibrate other substances.
-
PbF₄ is involved in oxidation reactions. Its strong oxidizing properties make it useful in these processes.
-
It’s used in chemical synthesis. PbF₄ helps create other compounds in the lab.
Safety and Handling of Lead Tetrafluoride
Given its toxicity, handling PbF₄ requires strict safety protocols. Here’s what you need to know.
-
Protective gear is essential. Gloves, goggles, and lab coats are a must.
-
Proper ventilation is crucial. Working with PbF₄ should be done in a well-ventilated area or fume hood.
-
Storage guidelines must be followed. PbF₄ should be stored in airtight containers away from moisture.
-
Disposal procedures are important. PbF₄ waste must be disposed of according to hazardous material regulations.
-
Training is required. Only trained personnel should handle PbF₄ to avoid accidents.
Interesting Facts about Lead Tetrafluoride
Beyond its scientific uses, PbF₄ has some quirky and lesser-known aspects.
-
PbF₄ can react explosively with certain substances. Mixing it with organic materials can be dangerous.
-
It’s part of a group called tetrahalides. These compounds contain one element bonded to four halogen atoms.
-
PbF₄ has been studied for decades. Scientists have been exploring its properties since the early 20th century.
-
It’s a rare compound. Not many labs work with PbF₄ due to its toxicity and handling challenges.
-
PbF₄ can decompose at high temperatures. When heated too much, it breaks down into lead and fluorine gas.
Environmental Impact of Lead Tetrafluoride
Like many chemicals, PbF₄ has environmental implications. Here’s what you should know.
-
PbF₄ is harmful to the environment. It can contaminate soil and water if not handled properly.
-
Lead pollution is a concern. Lead compounds can cause serious health issues in humans and animals.
-
Fluorine release is dangerous. Fluorine gas is highly reactive and can cause environmental damage.
-
Regulations exist. Governments have rules to control the use and disposal of PbF₄.
-
Research is ongoing. Scientists are studying ways to minimize the environmental impact of PbF₄.
Future of Lead Tetrafluoride
What does the future hold for PbF₄? Let’s explore some possibilities.
-
New applications are being explored. Scientists are looking for new ways to use PbF₄ in technology and industry.
-
Safer handling methods are in development. Researchers aim to make working with PbF₄ less hazardous.
-
Environmental solutions are a focus. Efforts are underway to reduce PbF₄’s environmental footprint.
-
Advanced research continues. Scientists are still uncovering new properties and potential uses for PbF₄.
-
Education and training are key. Future scientists need to learn about PbF₄ to continue exploring its potential.
Final Thoughts on Lead Tetrafluoride
Lead tetrafluoride, a compound with a unique set of properties, has intrigued scientists for years. Its chemical structure and reactivity make it a subject of ongoing research. While it’s not something you encounter daily, understanding its applications and potential hazards is crucial for those in the field of chemistry.
This compound’s ability to act as an oxidizing agent and its role in various industrial processes highlight its importance. However, handling it requires caution due to its toxicity and reactivity.
By exploring these 40 facts, you’ve gained a deeper insight into lead tetrafluoride’s characteristics and uses. Whether you’re a student, a professional chemist, or just curious, knowing about such compounds enriches your understanding of the chemical world. Stay curious and keep learning!
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
