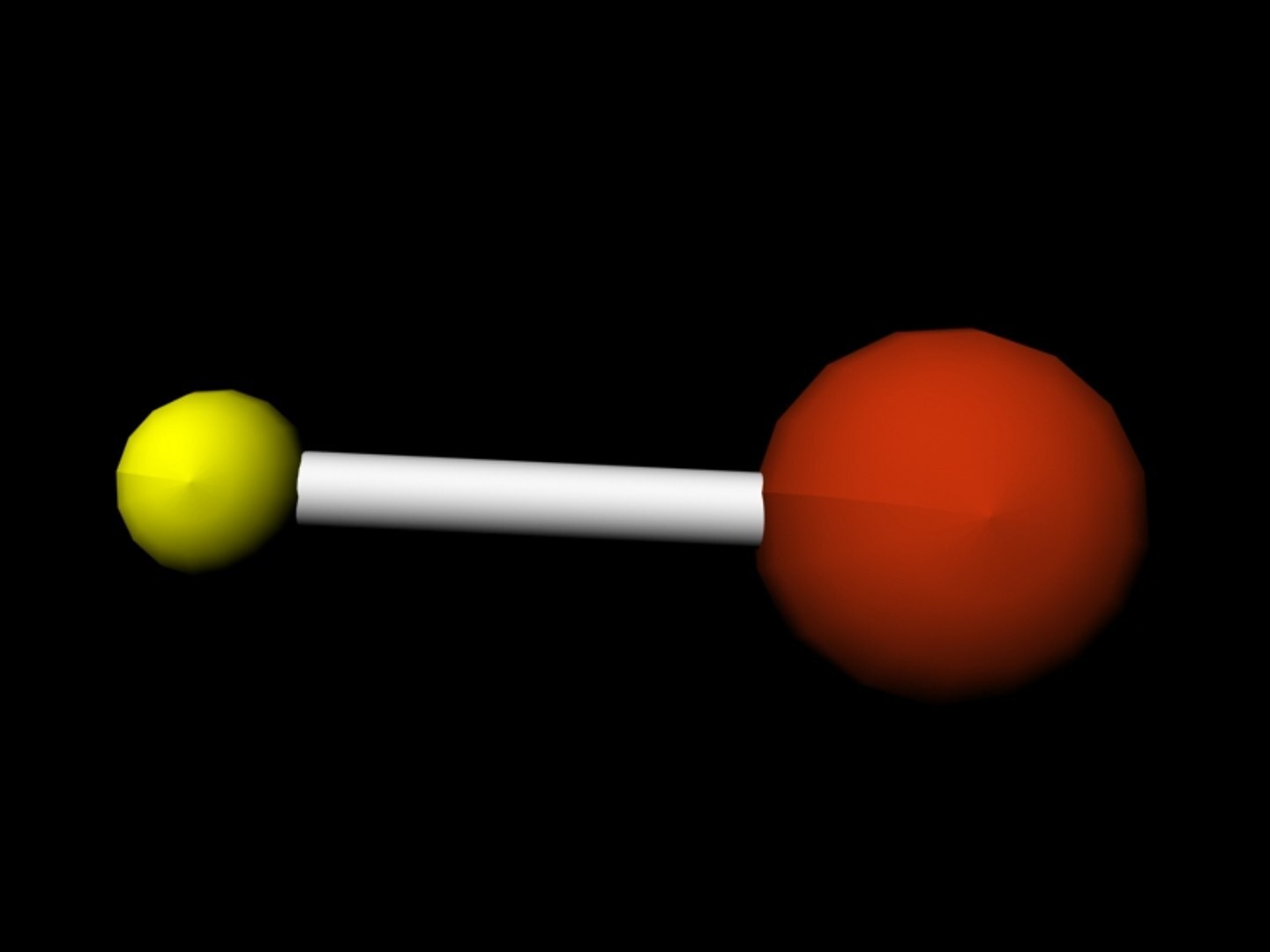
Hydrogen bromide is a chemical compound with the formula HBr. It's a colorless gas that dissolves in water to form hydrobromic acid, a strong acid with many industrial uses. Why should you care about hydrogen bromide? This compound plays a crucial role in various applications, from pharmaceuticals to organic synthesis. Understanding its properties and uses can help you grasp its importance in everyday products and processes. Whether you're a student, a science enthusiast, or just curious, these 40 facts about hydrogen bromide will give you a comprehensive overview of this fascinating compound. Get ready to dive into the world of HBr!
Key Takeaways:
- Hydrogen Bromide, a colorless gas with a sharp odor, is used in various industries for making acids, organic compounds, and flame retardants. It must be handled with care due to its corrosive nature.
- Hydrogen Bromide has a significant environmental impact, contributing to acid rain and ozone depletion. However, ongoing research is exploring its potential in green chemistry and renewable energy production.
What is Hydrogen Bromide?
Hydrogen Bromide (HBr) is a diatomic molecule consisting of hydrogen and bromine. It is a colorless gas with a sharp, irritating odor. When dissolved in water, it forms hydrobromic acid, a strong acid used in various industrial applications.
- Hydrogen Bromide is a diatomic molecule composed of one hydrogen atom and one bromine atom.
- It appears as a colorless gas under standard conditions.
- HBr has a sharp, irritating odor that can be quite pungent.
- When dissolved in water, it forms hydrobromic acid, a highly corrosive substance.
- Hydrobromic acid is a strong acid, similar in strength to hydrochloric acid.
Chemical Properties of Hydrogen Bromide
Hydrogen Bromide exhibits several interesting chemical properties that make it useful in various applications. Here are some key properties:
- HBr is a polar molecule due to the difference in electronegativity between hydrogen and bromine.
- It has a boiling point of -66.8°C (-88.2°F).
- The melting point of HBr is -86.9°C (-124.4°F).
- Hydrogen Bromide is highly soluble in water, forming hydrobromic acid.
- It can react with metals to form bromides and hydrogen gas.
Uses of Hydrogen Bromide
Hydrogen Bromide has a wide range of applications in different industries. Here are some of its primary uses:
- It is used in the production of inorganic bromides, such as sodium bromide and potassium bromide.
- HBr is employed in the synthesis of organic compounds, including pharmaceuticals and dyes.
- It serves as a catalyst in certain chemical reactions.
- Hydrogen Bromide is used in the manufacture of brominated flame retardants.
- It plays a role in the extraction of certain metals from their ores.
Safety and Handling of Hydrogen Bromide
Due to its corrosive nature, Hydrogen Bromide must be handled with care. Here are some safety considerations:
- HBr is highly corrosive and can cause severe burns upon contact with skin.
- Inhalation of Hydrogen Bromide gas can lead to respiratory irritation and damage.
- Proper protective equipment, such as gloves and goggles, should be worn when handling HBr.
- It should be stored in well-ventilated areas to prevent the buildup of gas.
- In case of a spill, neutralizing agents like sodium bicarbonate can be used to mitigate its effects.
Environmental Impact of Hydrogen Bromide
Hydrogen Bromide can have significant environmental effects if not managed properly. Here are some facts about its impact:
- HBr can contribute to acid rain when released into the atmosphere.
- It can react with ozone, leading to the depletion of the ozone layer.
- Hydrogen Bromide is toxic to aquatic life and can cause harm if it enters water bodies.
- Proper disposal methods are essential to prevent environmental contamination.
- Regulatory agencies monitor and control the emission of HBr to minimize its environmental impact.
Historical Facts about Hydrogen Bromide
Hydrogen Bromide has an interesting history in the field of chemistry. Here are some historical facts:
- HBr was first prepared in the early 19th century by French chemist Antoine Jérôme Balard.
- It was initially produced by reacting bromine with hydrogen.
- The industrial production of HBr began in the late 19th century.
- Early uses of Hydrogen Bromide included photographic processing.
- It played a role in the development of early organic chemistry.
Fun Facts about Hydrogen Bromide
Hydrogen Bromide isn't just about serious science; it has some fun and quirky aspects too. Here are a few:
- HBr is used in the production of certain types of candy to create a sour taste.
- It has been used in fire extinguishers due to its flame-retardant properties.
- Hydrogen Bromide can be used to etch glass, creating intricate designs.
- In the past, it was used as a sedative in medicine.
- HBr is sometimes used in chemical magic tricks to produce smoke or fog effects.
Future of Hydrogen Bromide
The future of Hydrogen Bromide looks promising with ongoing research and development. Here are some future prospects:
- Researchers are exploring its use in green chemistry to develop more sustainable processes.
- HBr could play a role in the production of renewable energy sources.
- Advances in nanotechnology may lead to new applications for Hydrogen Bromide.
- It is being studied for its potential in carbon capture and storage technologies.
- The development of safer handling methods will make its use more widespread in various industries.
Final Thoughts on Hydrogen Bromide
Hydrogen bromide, a fascinating compound, plays a significant role in various industries. From its use in organic synthesis to its application in pharmaceuticals, hydrogen bromide proves its versatility. Its properties, like being a strong acid and a potent reagent, make it indispensable in many chemical reactions. However, handling it requires caution due to its corrosive nature and potential health hazards. Always prioritize safety when working with this compound. Understanding these facts about hydrogen bromide not only broadens your knowledge but also highlights the importance of this chemical in everyday applications. Whether you're a student, a professional, or just curious, knowing these details can be quite enlightening. Stay informed, stay safe, and appreciate the wonders of chemistry.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
