Cyanogen bromide might sound like something out of a sci-fi movie, but it's a real chemical with some fascinating uses. What is cyanogen bromide? It's a compound made from carbon, nitrogen, and bromine, often used in biochemistry and molecular biology. This chemical is known for its ability to break down proteins, making it a handy tool for scientists studying protein structures. But that's not all! Cyanogen bromide also plays a role in synthesizing certain pharmaceuticals and even in fumigation. However, it's important to handle it with care due to its toxic nature. Ready to learn more? Let's dive into 40 intriguing facts about cyanogen bromide!
Key Takeaways:
- Cyanogen Bromide is a versatile chemical compound used in biochemistry and organic synthesis. It's toxic, but crucial for protein research, DNA sequencing, and drug development.
- Handle cyanogen bromide with caution! It's toxic, pungent, and can harm the environment. But it's also valuable for scientific research and material science.
What is Cyanogen Bromide?
Cyanogen bromide, often abbreviated as CNBr, is a chemical compound with various applications in biochemistry and organic synthesis. Here are some intriguing facts about this versatile substance.
-
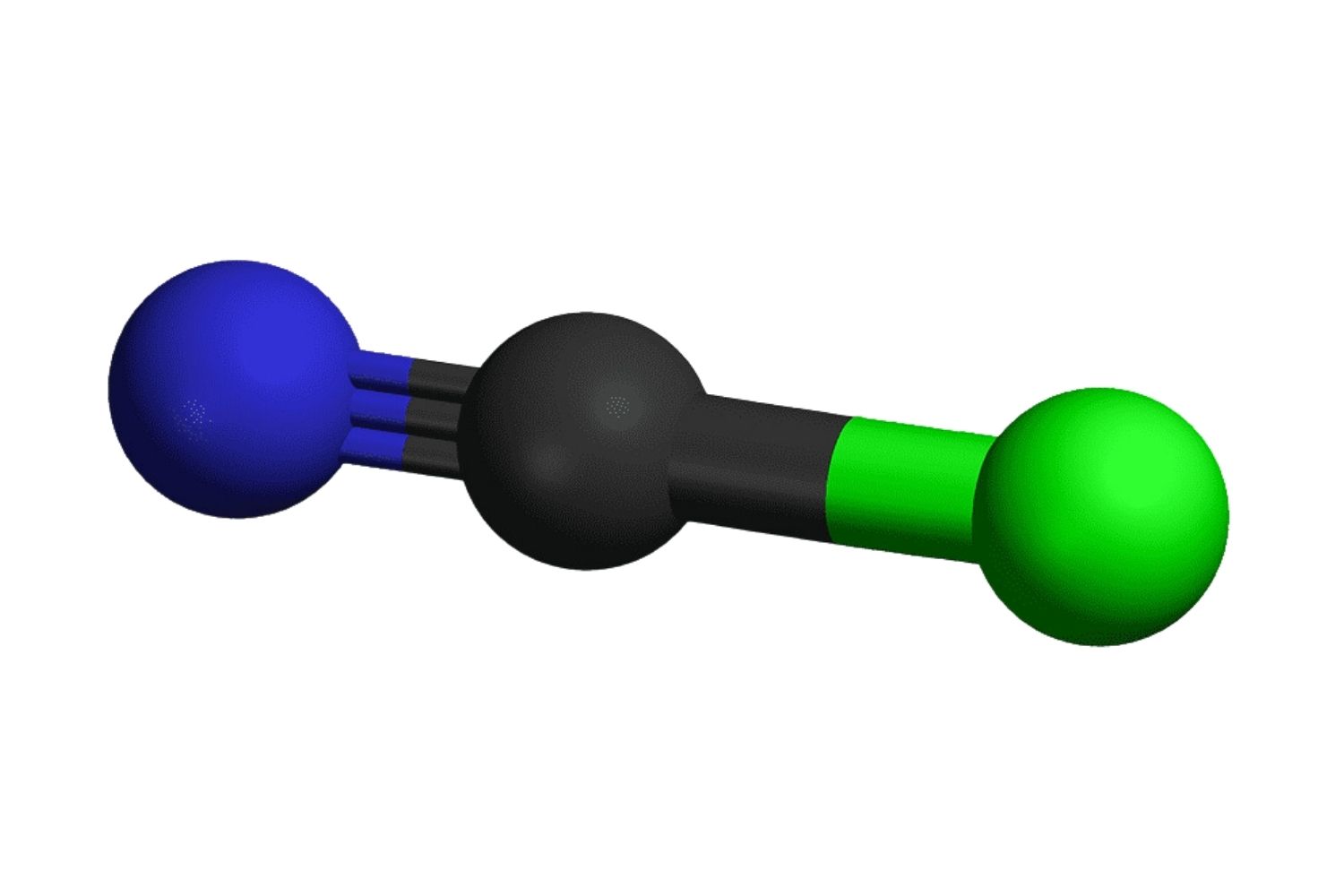
Chemical Formula: CNBr's chemical formula is BrCN, indicating it contains bromine, carbon, and nitrogen.
-
Appearance: It appears as a white crystalline solid, making it easy to identify in a lab setting.
-
Odor: CNBr has a pungent smell, similar to that of cyanide, which can be quite strong.
-
Solubility: It dissolves well in water, ethanol, and acetone, making it versatile for different chemical reactions.
-
Melting Point: CNBr melts at 52°C (126°F), which is relatively low for a solid compound.
Uses in Biochemistry
Cyanogen bromide is widely used in biochemistry, particularly in protein research and peptide synthesis.
-
Protein Cleavage: CNBr is used to cleave proteins at methionine residues, aiding in protein sequencing.
-
Peptide Synthesis: It helps in synthesizing peptides by forming peptide bonds between amino acids.
-
Enzyme Inactivation: CNBr can inactivate enzymes by modifying their active sites.
-
Affinity Chromatography: It is used to activate agarose beads for affinity chromatography, a technique to purify proteins.
-
Immobilization of Proteins: CNBr helps immobilize proteins on solid supports for various biochemical assays.
Safety and Handling
Handling cyanogen bromide requires caution due to its toxic and hazardous nature.
-
Toxicity: CNBr is highly toxic if inhaled, ingested, or absorbed through the skin.
-
Protective Gear: Always wear gloves, goggles, and lab coats when handling CNBr.
-
Ventilation: Use CNBr in a well-ventilated area or fume hood to avoid inhaling fumes.
-
Storage: Store CNBr in a cool, dry place away from light and moisture to prevent decomposition.
-
First Aid: In case of exposure, seek immediate medical attention and follow proper first aid procedures.
Chemical Reactions
Cyanogen bromide participates in various chemical reactions, making it valuable in organic synthesis.
-
Nucleophilic Substitution: CNBr undergoes nucleophilic substitution reactions, replacing the bromine atom with other nucleophiles.
-
Cyclization Reactions: It can induce cyclization reactions, forming cyclic compounds from linear precursors.
-
Dehydration Reactions: CNBr acts as a dehydrating agent, removing water molecules from organic compounds.
-
Oxidation Reactions: It can oxidize certain organic molecules, changing their oxidation state.
-
Addition Reactions: CNBr can add to double bonds in unsaturated compounds, forming new products.
Historical Context
Understanding the history of cyanogen bromide provides insight into its development and applications.
-
Discovery: CNBr was first synthesized in the 19th century by chemists exploring cyanogen compounds.
-
Early Uses: Initially, it was used in organic synthesis and as a fumigant.
-
Biochemical Applications: Its use in biochemistry became prominent in the mid-20th century with advancements in protein research.
-
Industrial Production: CNBr is now produced on an industrial scale for various scientific and commercial applications.
-
Regulations: Due to its toxicity, CNBr is regulated by safety guidelines to ensure proper handling and disposal.
Environmental Impact
The environmental impact of cyanogen bromide is an important consideration for its use and disposal.
-
Decomposition: CNBr decomposes in water, releasing toxic cyanide ions, which can harm aquatic life.
-
Air Pollution: If released into the air, CNBr can contribute to air pollution and pose health risks.
-
Waste Disposal: Proper disposal methods are essential to prevent environmental contamination.
-
Biodegradability: CNBr is not biodegradable, so it persists in the environment if not properly managed.
-
Regulatory Measures: Environmental agencies have guidelines for the safe disposal of CNBr to minimize its impact.
Interesting Facts
Here are some lesser-known yet fascinating facts about cyanogen bromide.
-
Crystallography: CNBr crystals are used in X-ray crystallography to determine the structure of proteins.
-
Historical Use: During World War I, CNBr was considered for use as a chemical weapon but was never deployed.
-
Synthesis: CNBr can be synthesized by reacting bromine with cyanogen gas or sodium cyanide.
-
Stability: It is relatively stable under normal conditions but can decompose when exposed to light or heat.
-
Reactivity: CNBr is highly reactive with nucleophiles, making it useful in various chemical transformations.
Applications in Research
Cyanogen bromide continues to be a valuable tool in scientific research.
-
DNA Sequencing: CNBr is used in DNA sequencing techniques to cleave DNA at specific sites.
-
Protein Engineering: It aids in protein engineering by modifying protein structures and functions.
-
Bioconjugation: CNBr is used in bioconjugation to link biomolecules for various applications.
-
Drug Development: Researchers use CNBr in drug development to synthesize and modify pharmaceutical compounds.
-
Material Science: CNBr is used in material science to create new materials with unique properties.
The Final Word on Cyanogen Bromide
Cyanogen bromide, a fascinating compound, has a wide range of applications. From its role in protein sequencing to its use in organic synthesis, this chemical proves invaluable in scientific research. Despite its usefulness, it’s crucial to handle it with care due to its toxic nature. Always follow safety guidelines when working with this substance.
Understanding cyanogen bromide’s properties and uses can enhance your knowledge of chemistry and its practical applications. Whether you’re a student, researcher, or just curious, knowing these facts can provide a solid foundation for further exploration.
Remember, safety first! Proper handling and respect for its potential hazards ensure that cyanogen bromide remains a helpful tool in various fields. Keep learning and stay curious about the world of chemistry.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
