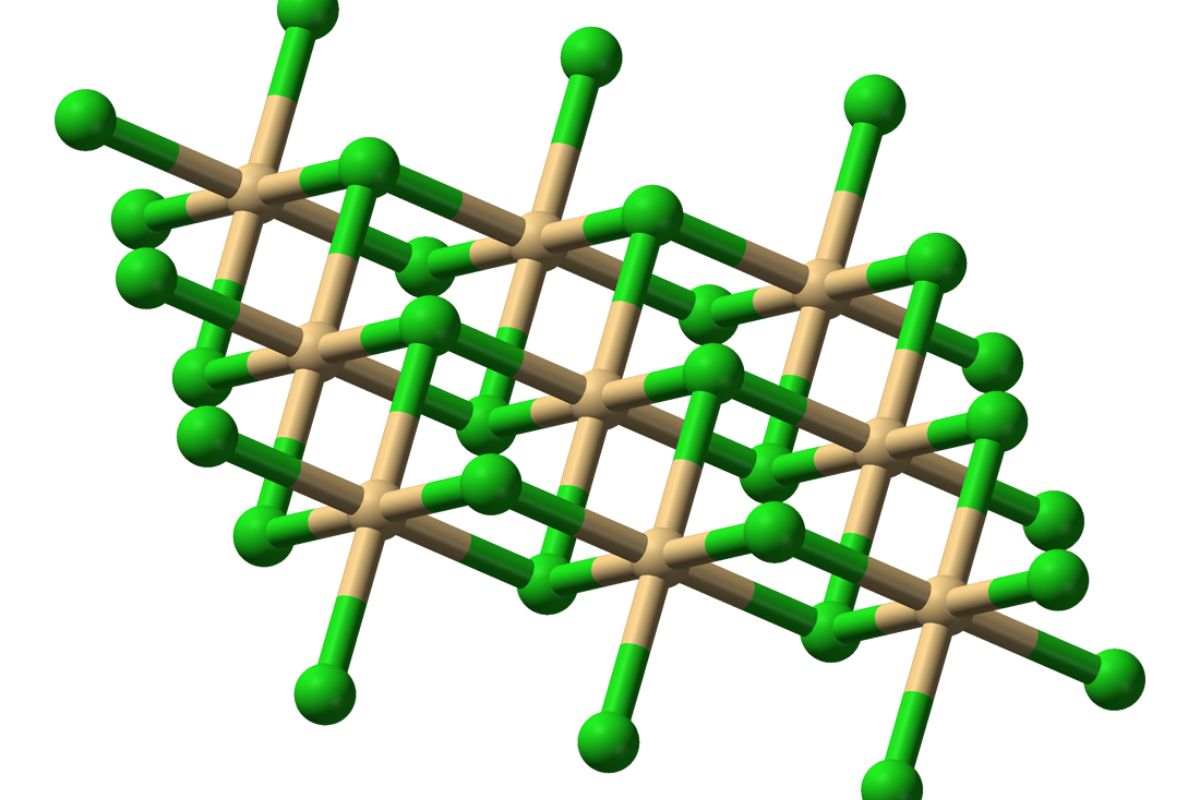
What is Cadmium Bromide? Cadmium bromide is a chemical compound with the formula CdBr₂. This compound is a white crystalline solid that dissolves easily in water. It's often used in photography, lithography, and as a chemical reagent. Is cadmium bromide dangerous? Yes, it can be hazardous. Exposure to cadmium bromide can lead to serious health issues, including lung and kidney damage. It's crucial to handle it with care, using protective gear. Where is cadmium bromide found? It's not something you find in nature. Instead, it's manufactured for industrial and scientific purposes. Why is cadmium bromide important? Despite its risks, cadmium bromide plays a vital role in various industries, especially in creating specialized materials and conducting research. Understanding its properties and uses can help us appreciate its significance while respecting its potential dangers.
Key Takeaways:
- Cadmium bromide, with the chemical formula CdBr₂, is a versatile compound used in photography, lithography, and chemical synthesis. It has unique properties but requires careful handling due to its toxicity and environmental impact.
- Despite its toxicity, cadmium bromide is still used in modern applications such as optical devices, semiconductor industry, and catalysis. However, research is ongoing to find alternative compounds and develop methods for environmental remediation.
What is Cadmium Bromide?
Cadmium bromide is a chemical compound with the formula CdBr₂. It's a white, crystalline solid that dissolves in water. This compound is used in photography, lithography, and as a reagent in chemical synthesis. Let's explore some fascinating facts about cadmium bromide.
-
Chemical Formula: Cadmium bromide's chemical formula is CdBr₂, indicating it contains one cadmium atom and two bromine atoms.
-
Appearance: This compound appears as a white crystalline solid, making it easy to identify in its pure form.
-
Solubility: Cadmium bromide dissolves well in water, which is useful for various chemical reactions and applications.
-
Uses in Photography: It plays a role in the photographic industry, particularly in the preparation of photographic emulsions.
-
Lithography Applications: In lithography, cadmium bromide is used to create detailed images on printing plates.
-
Chemical Reagent: As a reagent, it helps in synthesizing other chemical compounds, showcasing its versatility.
Physical and Chemical Properties
Understanding the physical and chemical properties of cadmium bromide helps in grasping its behavior in different environments.
-
Melting Point: Cadmium bromide has a melting point of approximately 568°C, indicating its stability at high temperatures.
-
Boiling Point: Its boiling point is around 844°C, further demonstrating its thermal resilience.
-
Density: The density of cadmium bromide is about 5.192 g/cm³, making it relatively dense compared to other compounds.
-
Molecular Weight: With a molecular weight of 272.22 g/mol, it is considered a heavy compound.
-
Crystal Structure: It forms a monoclinic crystal structure, which is a common arrangement for many inorganic compounds.
-
Reactivity: Cadmium bromide reacts with strong acids and bases, which can lead to the formation of other compounds.
Safety and Environmental Impact
Safety is crucial when handling chemical compounds, and cadmium bromide is no exception. Its impact on health and the environment is significant.
-
Toxicity: Cadmium bromide is toxic if ingested or inhaled, requiring careful handling and storage.
-
Carcinogenic Potential: It is considered a potential carcinogen, meaning it could contribute to cancer development over time.
-
Environmental Hazard: This compound poses a risk to aquatic life, necessitating proper disposal to prevent environmental contamination.
-
Protective Measures: When working with cadmium bromide, using protective gear like gloves and masks is essential to minimize exposure.
-
Regulations: Various regulations govern the use and disposal of cadmium bromide to protect human health and the environment.
Historical and Scientific Context
Cadmium bromide has a rich history and scientific significance that adds to its intrigue.
-
Discovery: Cadmium was discovered in 1817 by Friedrich Stromeyer, but cadmium bromide's specific history is less documented.
-
Scientific Studies: Numerous studies have been conducted to understand its properties and potential applications better.
-
Role in Research: It serves as a model compound in research to study cadmium's behavior in various chemical reactions.
-
Historical Uses: Historically, cadmium bromide was used in medicine, although this practice has largely been discontinued due to toxicity concerns.
-
Advancements: Advances in chemistry have led to safer alternatives, reducing the reliance on cadmium bromide in some applications.
Interesting Facts and Trivia
Beyond its scientific and industrial uses, cadmium bromide has some intriguing aspects worth noting.
-
Color Change: When heated, cadmium bromide can change color, a property that can be used in certain applications.
-
Luminescence: It exhibits luminescent properties under specific conditions, making it interesting for optical studies.
-
Cadmium Source: Cadmium, the metal in cadmium bromide, is often sourced from zinc ores as a byproduct.
-
Bromine Connection: Bromine, part of cadmium bromide, is a halogen known for its reactivity and use in flame retardants.
-
Cadmium's Name Origin: The name "cadmium" comes from the Latin word "cadmia," meaning calamine, a zinc ore.
-
Bromide Ions: Bromide ions in cadmium bromide are also found in many other compounds, including some medications.
Cadmium Bromide in Modern Applications
Despite its toxicity, cadmium bromide still finds use in modern applications, thanks to its unique properties.
-
Optical Devices: It is used in the production of certain optical devices due to its refractive properties.
-
Semiconductor Industry: Cadmium bromide plays a role in the semiconductor industry, particularly in the development of specific types of detectors.
-
Catalysis: It acts as a catalyst in some chemical reactions, speeding up processes without being consumed.
-
Research Tool: Scientists use cadmium bromide as a tool to study the effects of cadmium in biological systems.
-
Synthesis of Nanomaterials: It is involved in the synthesis of cadmium-based nanomaterials, which have potential applications in technology.
Handling and Storage
Proper handling and storage of cadmium bromide are crucial to ensure safety and maintain its integrity.
-
Storage Conditions: It should be stored in a cool, dry place away from incompatible substances to prevent unwanted reactions.
-
Handling Precautions: Using fume hoods and proper ventilation is important when working with cadmium bromide to avoid inhalation.
-
Spill Management: In case of a spill, it is important to clean it up immediately using appropriate protective equipment.
-
Labeling: Proper labeling of containers is essential to prevent accidental misuse or exposure.
-
Disposal: Disposal of cadmium bromide must comply with local regulations to minimize environmental impact.
Future Prospects and Research
Research continues to explore new ways to utilize cadmium bromide while minimizing its risks.
-
Alternative Compounds: Scientists are investigating alternative compounds that can replace cadmium bromide in certain applications.
-
Environmental Remediation: Research is ongoing to develop methods for remediating cadmium contamination in the environment.
Cadmium Bromide: A Quick Recap
Cadmium bromide, a chemical compound with the formula CdBr₂, plays a significant role in various industries. It's used in photography, lithography, and as a catalyst in organic synthesis. Despite its usefulness, it's crucial to handle it with care due to its toxicity. Exposure can lead to serious health issues, so proper safety measures are essential when working with this compound.
Understanding its properties and applications helps in appreciating its importance while respecting its potential hazards. Always prioritize safety and environmental considerations when dealing with cadmium bromide. Whether you're a student, researcher, or industry professional, knowing these facts ensures you're well-informed about this intriguing compound.
Remember, knowledge is power, especially when it comes to handling chemicals safely. Stay curious, stay safe, and keep exploring the fascinating world of chemistry.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
