Bromine Pentafluoride, a chemical compound with the formula BrF5, is a powerful fluorinating agent. What makes Bromine Pentafluoride so intriguing? Its ability to react with nearly everything, even substances that typically resist chemical reactions, sets it apart. This compound is a colorless liquid at room temperature, but it can be quite dangerous due to its highly corrosive nature. Handling it requires extreme caution, as it can cause severe burns upon contact with skin or other materials. Despite its hazards, Bromine Pentafluoride plays a crucial role in various industrial applications, including rocket propellants and chemical synthesis. Its unique properties make it a subject of interest for scientists and industries alike. Understanding its behavior and applications can provide insights into the fascinating world of chemical reactions and industrial processes. Whether you're a chemistry enthusiast or just curious, Bromine Pentafluoride offers a glimpse into the power and complexity of chemical compounds.
Key Takeaways:
- Bromine pentafluoride is a powerful chemical with uses in rocket propellants and uranium processing, but it requires careful handling due to its corrosive and toxic nature.
- Its discovery has contributed to our understanding of halogen chemistry, and its high reactivity makes it valuable for research and industrial applications, but it must be handled with extreme caution.
What is Bromine Pentafluoride?
Bromine pentafluoride is a chemical compound with the formula BrF₅. It's a colorless liquid, known for its strong oxidizing properties and its ability to react with many substances. Let's explore some intriguing facts about this compound.
-
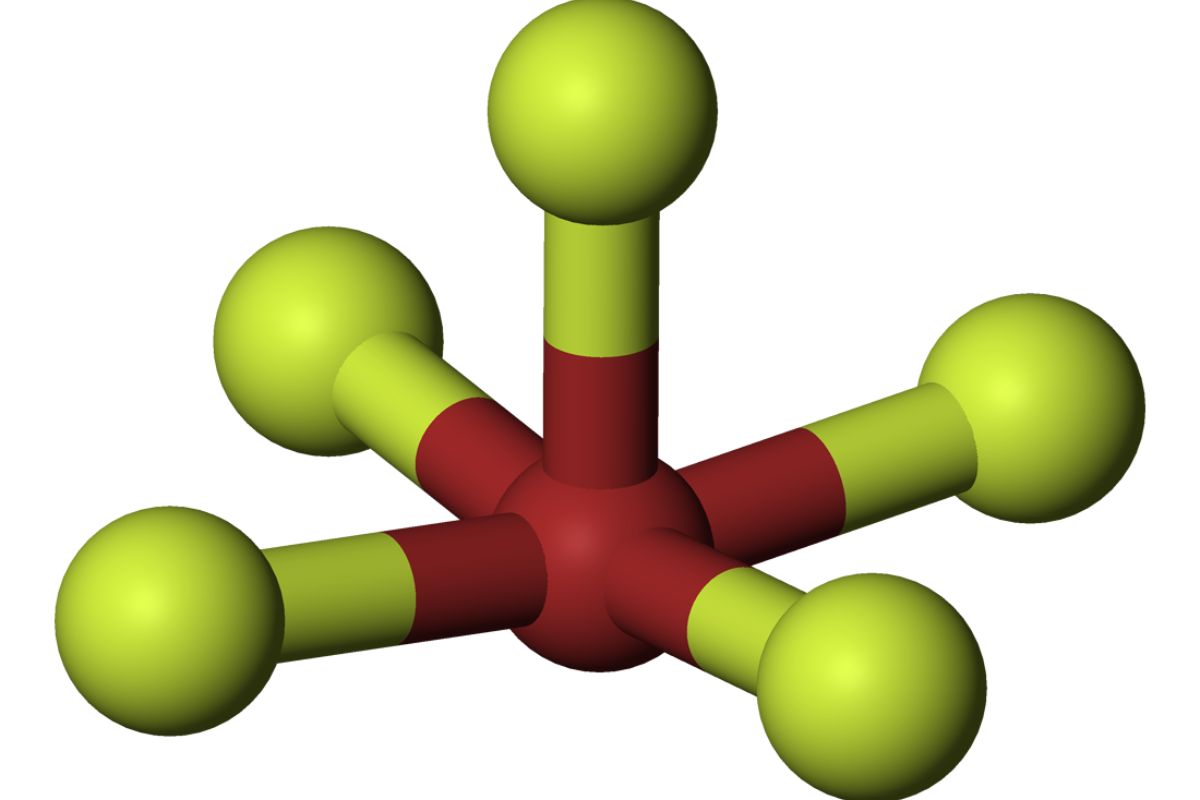
Chemical Formula: BrF₅ is the chemical formula for bromine pentafluoride. It consists of one bromine atom and five fluorine atoms.
-
Discovery: This compound was first synthesized in 1931 by American chemist Paul Lebeau. He achieved this by reacting bromine with fluorine gas.
-
Physical State: At room temperature, bromine pentafluoride is a colorless liquid. It has a pungent odor and is highly corrosive.
-
Boiling Point: The boiling point of bromine pentafluoride is 40.25°C (104.45°F), making it a liquid at room temperature.
-
Density: It has a density of 2.466 g/cm³, which is relatively high compared to water.
Uses of Bromine Pentafluoride
Bromine pentafluoride has several applications, particularly in the field of chemistry and industry. Here are some of its uses:
-
Fluorinating Agent: It's primarily used as a fluorinating agent in chemical reactions, helping to introduce fluorine atoms into other compounds.
-
Rocket Propellant: In the past, it was considered as a potential rocket propellant due to its high reactivity and ability to release energy.
-
Uranium Processing: BrF₅ is used in the processing of uranium, particularly in the conversion of uranium compounds to uranium hexafluoride, an important step in nuclear fuel production.
-
Chemical Synthesis: It plays a role in the synthesis of various organic and inorganic compounds, especially those requiring fluorination.
Safety and Handling
Due to its highly reactive nature, bromine pentafluoride requires careful handling. Here are some safety facts:
-
Corrosive Nature: BrF₅ is highly corrosive and can cause severe burns upon contact with skin or eyes.
-
Toxicity: Inhalation of its vapors can be harmful, causing respiratory issues and other health problems.
-
Protective Gear: Handling this compound requires the use of protective gear, including gloves, goggles, and face shields.
-
Storage: It should be stored in containers made of materials resistant to fluorine, such as nickel or monel.
Chemical Reactions
Bromine pentafluoride is known for its vigorous reactions with various substances. Here are some reaction facts:
-
Water Reaction: When it comes into contact with water, it reacts violently, producing hydrofluoric acid and bromic acid.
-
Metal Reactions: It reacts with many metals, often forming metal fluorides and releasing heat.
-
Organic Compounds: BrF₅ can react with organic compounds, often leading to the substitution of hydrogen atoms with fluorine.
-
Non-Metal Reactions: It can also react with non-metals like sulfur and phosphorus, forming corresponding fluorides.
Interesting Facts
Beyond its chemical properties and uses, bromine pentafluoride has some fascinating aspects:
-
Molecular Geometry: The molecule has a square pyramidal shape, with the bromine atom at the center and fluorine atoms at the corners.
-
Oxidation State: In BrF₅, bromine is in the +5 oxidation state, which is relatively high for this element.
-
Reactivity: Its high reactivity is due to the presence of fluorine, the most electronegative element, which makes BrF₅ a strong oxidizing agent.
-
Color Change: While it's colorless in its pure form, it can appear yellowish when impure or when exposed to light.
-
Environmental Impact: Due to its reactivity, it can have significant environmental impacts if released, necessitating careful containment and disposal.
-
Industrial Production: It's produced industrially by direct fluorination of bromine, a process that requires careful control to prevent explosions.
-
Laboratory Use: In research settings, it's used to study fluorination reactions and the properties of fluorinated compounds.
-
Historical Significance: Its discovery and subsequent study have contributed to the understanding of halogen chemistry and the development of fluorination techniques.
-
Handling Precautions: Due to its hazardous nature, only trained personnel should handle bromine pentafluoride, following strict safety protocols.
-
Chemical Stability: While reactive, it remains stable under controlled conditions, making it useful in various chemical processes.
-
Decomposition: Upon decomposition, it releases toxic gases, including fluorine and bromine, which require careful management.
-
Compatibility: It's incompatible with many materials, including glass and certain plastics, which can be corroded by its action.
-
Emergency Measures: In case of a spill or leak, specific emergency measures must be in place to contain and neutralize the compound safely.
-
Regulatory Status: Due to its potential hazards, its use and handling are subject to regulations and guidelines to ensure safety.
-
Research Applications: It's used in research to explore new fluorination methods and to synthesize novel fluorinated compounds.
-
Chemical Bonding: The bonds between bromine and fluorine in BrF₅ are strong, contributing to its stability and reactivity.
-
Industrial Challenges: Its production and use pose challenges due to the need for specialized equipment and safety measures.
-
Environmental Regulations: Its environmental impact is regulated to prevent contamination and ensure safe disposal.
-
Innovative Uses: Researchers continue to explore new applications for bromine pentafluoride in various fields, including materials science and energy.
-
Global Production: While not produced in large quantities, it's manufactured in specialized facilities around the world.
-
Market Demand: Its demand is driven by its applications in fluorination and nuclear industries, among others.
-
Future Prospects: Ongoing research aims to develop safer and more efficient methods for using bromine pentafluoride in various applications.
-
Educational Importance: Studying bromine pentafluoride provides insights into chemical reactivity, safety, and the role of fluorine in chemistry.
Final Thoughts on Bromine Pentafluoride
Bromine Pentafluoride is a fascinating compound with a wide range of applications and unique properties. Its role as a powerful oxidizing agent makes it invaluable in industries like rocket propulsion and chemical synthesis. However, its reactivity and toxicity demand careful handling and storage. Understanding its chemical behavior helps in harnessing its potential while ensuring safety.
This compound's ability to react with a variety of substances highlights its versatility, but also underscores the importance of proper safety protocols. Researchers and industries continue to explore its capabilities, pushing the boundaries of what's possible in chemical reactions.
In essence, Bromine Pentafluoride is a reminder of the delicate balance between innovation and safety in the world of chemistry. As we continue to learn more about it, the potential for new discoveries remains vast and exciting.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
