Iron–sulfur clusters are tiny but mighty players in the world of biochemistry. These clusters, made of iron and sulfur atoms, are found in many proteins and enzymes. They help with electron transfer, which is crucial for energy production in cells. Without them, life as we know it wouldn't exist. Iron–sulfur clusters are involved in processes like DNA repair and regulation of gene expression. They even play a role in sensing environmental changes. Despite their small size, these clusters have a big impact on living organisms. Ready to learn more? Here are 35 fascinating facts about iron–sulfur clusters.
Key Takeaways:
- Iron–sulfur clusters are tiny but mighty structures found in all forms of life, playing crucial roles in processes like respiration, photosynthesis, and DNA repair.
- Deficiencies in iron–sulfur clusters can lead to serious health issues, including genetic disorders, mitochondrial diseases, anemia, and neurodegenerative conditions.
What are Iron–Sulfur Clusters?
Iron–sulfur clusters are small, versatile structures found in many proteins. They play crucial roles in various biological processes, including electron transfer and enzyme catalysis. Here are some fascinating facts about these tiny powerhouses.
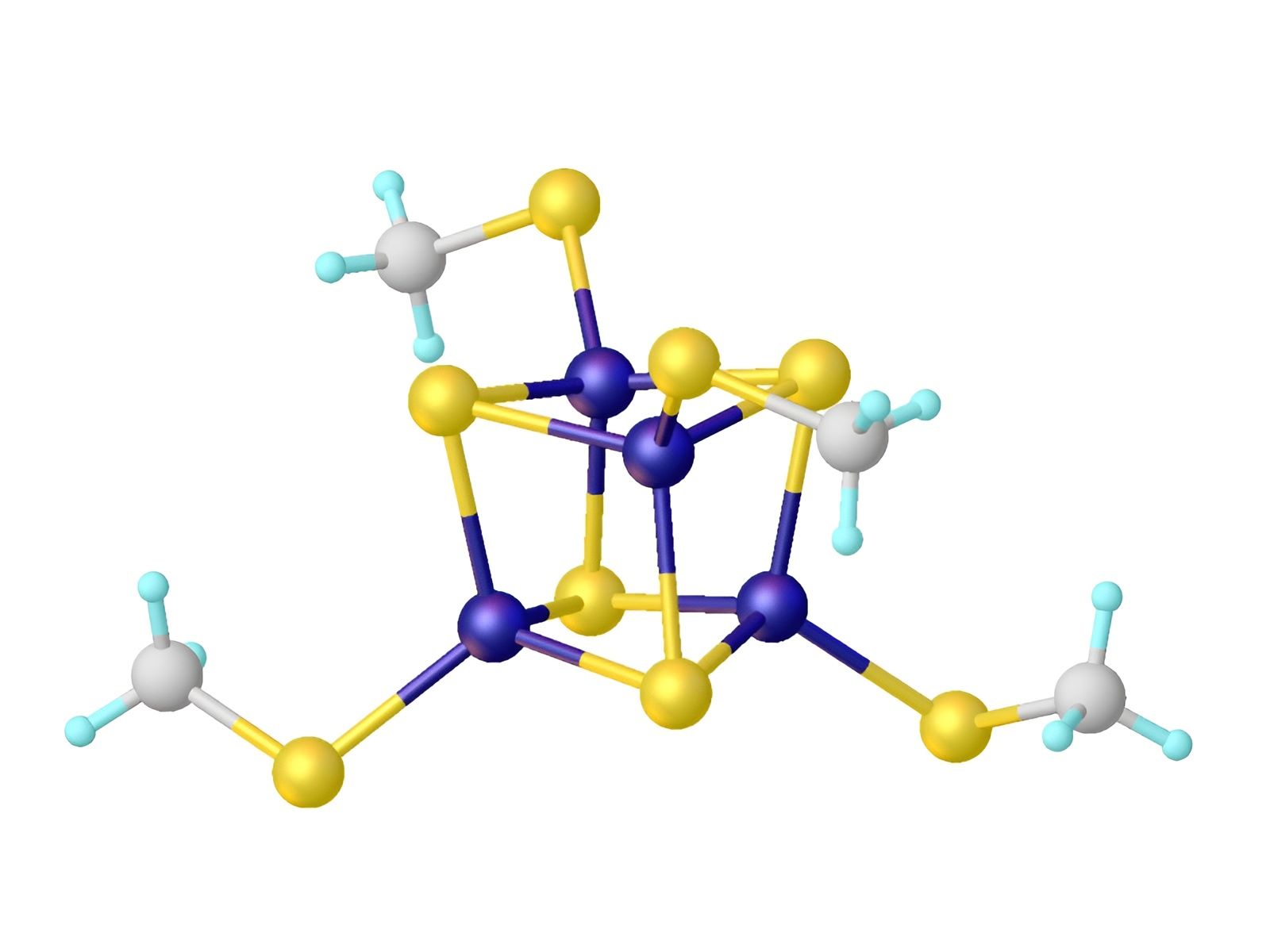
- Iron–sulfur clusters consist of iron and sulfur atoms arranged in specific geometric patterns.
- These clusters are found in all forms of life, from bacteria to humans.
- They are essential for the function of many enzymes, including those involved in DNA repair.
- Iron–sulfur clusters can exist in different forms, such as [2Fe-2S], [3Fe-4S], and [4Fe-4S].
- The clusters can change their oxidation states, allowing them to participate in redox reactions.
Biological Importance of Iron–Sulfur Clusters
Iron–sulfur clusters are vital for many biological functions. They help in processes like respiration, photosynthesis, and nitrogen fixation. Let's explore some key roles they play.
- In mitochondria, iron–sulfur clusters are part of the electron transport chain, which generates ATP.
- They are involved in the synthesis of iron-sulfur proteins, which are crucial for cellular metabolism.
- Iron–sulfur clusters help in the regulation of gene expression by acting as sensors for oxygen and iron levels.
- They are essential for the function of ribonucleotide reductase, an enzyme needed for DNA synthesis.
- In plants, iron–sulfur clusters are involved in photosynthesis, helping convert sunlight into chemical energy.
How Iron–Sulfur Clusters are Formed
The formation of iron–sulfur clusters is a complex process that involves multiple steps and proteins. Here's a look at how these clusters come to be.
- The process begins with the assembly of iron and sulfur atoms into a basic cluster.
- Specific proteins, known as scaffold proteins, assist in the initial assembly of the cluster.
- Once formed, the cluster is transferred to target proteins by carrier proteins.
- The entire process is tightly regulated to ensure proper cluster formation and function.
- Defects in cluster formation can lead to various diseases, including neurodegenerative disorders.
Diseases Linked to Iron–Sulfur Cluster Deficiency
Iron–sulfur cluster deficiencies can have severe consequences for health. Here are some diseases associated with these deficiencies.
- Friedreich's ataxia is a genetic disorder caused by impaired iron–sulfur cluster formation.
- Deficiencies in iron–sulfur clusters can lead to mitochondrial diseases, affecting energy production.
- Some forms of anemia are linked to problems with iron–sulfur cluster assembly.
- Neurodegenerative diseases, such as Parkinson's, have been associated with iron–sulfur cluster dysfunction.
- Iron–sulfur cluster deficiencies can also affect the immune system, leading to increased susceptibility to infections.
Interesting Facts about Iron–Sulfur Clusters
Beyond their biological importance, iron–sulfur clusters have some intriguing properties and uses. Here are a few interesting tidbits.
- Iron–sulfur clusters can act as molecular switches, changing their structure in response to environmental changes.
- They are used in some industrial processes, such as the synthesis of ammonia.
- Iron–sulfur clusters have been studied for their potential use in nanotechnology and materials science.
- They can be artificially synthesized in the lab, allowing scientists to study their properties in detail.
- Some bacteria use iron–sulfur clusters to detoxify harmful substances, such as heavy metals.
Research and Future Directions
Research on iron–sulfur clusters continues to uncover new insights and potential applications. Here are some exciting areas of ongoing research.
- Scientists are exploring the use of iron–sulfur clusters in developing new antibiotics.
- Research is being conducted on the role of iron–sulfur clusters in aging and age-related diseases.
- There is interest in using iron–sulfur clusters to develop new catalysts for chemical reactions.
- Studies are investigating how iron–sulfur clusters can be used to improve crop yields and agricultural productivity.
- Researchers are looking at ways to harness the properties of iron–sulfur clusters for renewable energy technologies.
Fun Facts about Iron–Sulfur Clusters
Let's wrap up with some fun and quirky facts about iron–sulfur clusters that you might not know.
- Iron–sulfur clusters can give proteins a yellow or brown color due to their unique structure.
- Some ancient bacteria used iron–sulfur clusters to survive in extreme environments, such as deep-sea vents.
- The study of iron–sulfur clusters has helped scientists understand the early evolution of life on Earth.
- Iron–sulfur clusters can be found in some of the oldest known enzymes, dating back billions of years.
- The unique properties of iron–sulfur clusters make them a subject of fascination for chemists and biologists alike.
Iron–Sulfur Clusters: The Final Word
Iron–sulfur clusters are tiny yet mighty components in living organisms. They play crucial roles in electron transfer, enzyme function, and even DNA repair. Without these clusters, many biological processes would grind to a halt. Understanding their structure and function helps scientists develop new medical treatments and technologies.
These clusters are found in a variety of organisms, from bacteria to humans. Their versatility and importance can't be overstated. Research continues to uncover new aspects of their function, promising exciting advancements in science and medicine.
So next time you think about the building blocks of life, remember the iron–sulfur clusters. They may be small, but their impact is enormous. Keep an eye on this field; it's bound to bring more fascinating discoveries in the future.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
