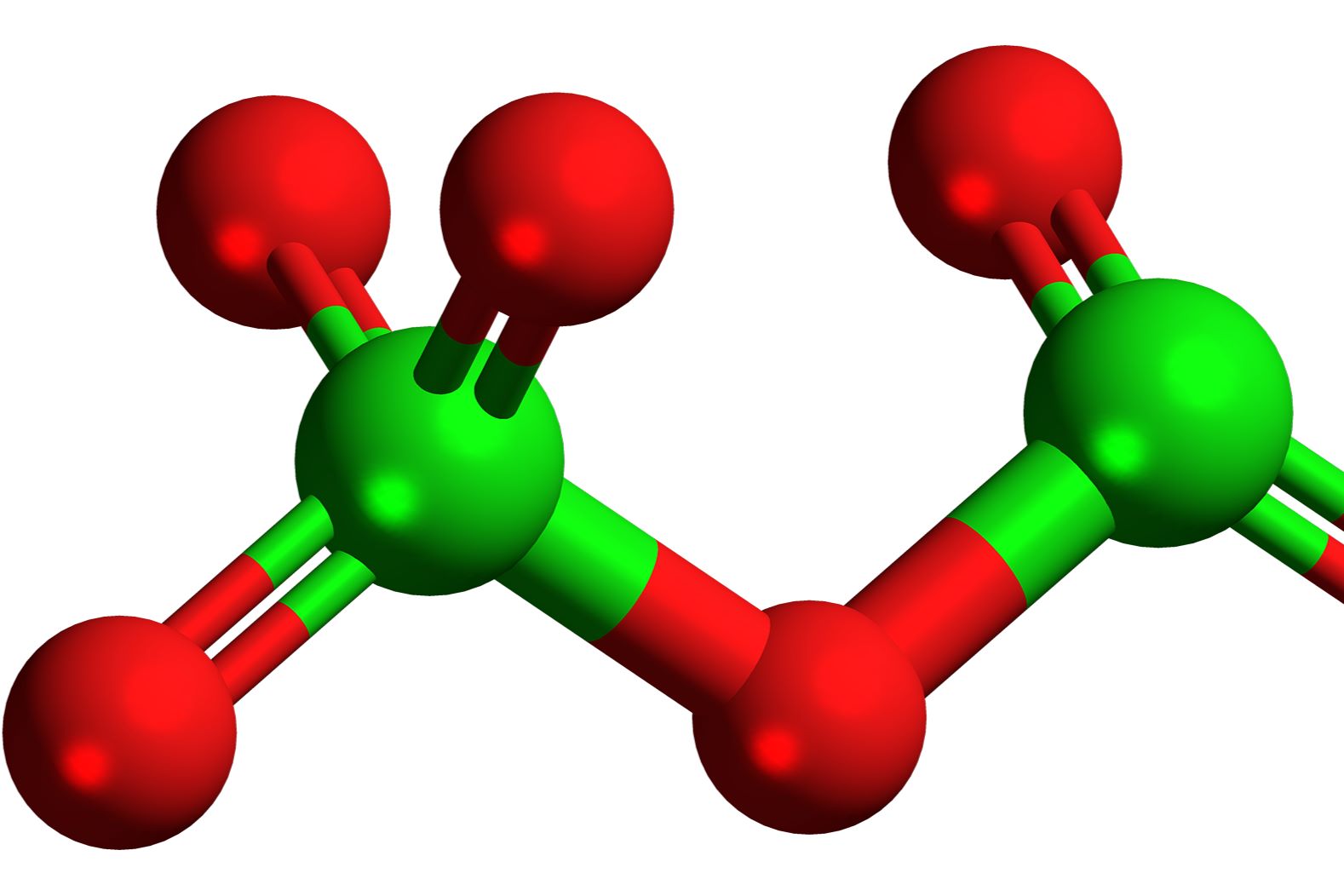
Dichlorine hexoxide might sound like something out of a sci-fi movie, but it's a real chemical compound with some pretty interesting facts. This compound, also known as Cl2O6, is a chlorine oxide that plays a role in various chemical reactions. Ever wondered what makes it so special? For starters, it's a powerful oxidizing agent, which means it can easily accept electrons from other substances. This property makes it useful in different industrial processes. But there's more! Dichlorine hexoxide is also quite unstable, decomposing into other compounds under certain conditions. Curious about its uses, properties, and more? Let's dive into 30 fascinating facts about this intriguing compound.
Key Takeaways:
- Dichlorine Hexoxide, also known as chlorine dioxide, is a powerful and unstable compound with various industrial uses, but it requires careful handling due to its toxicity and environmental impact.
- Ongoing research is uncovering new potential uses for dichlorine hexoxide, from antimicrobial coatings to environmental pollutant degradation, showing its versatility and potential for positive impact.
What is Dichlorine Hexoxide?
Dichlorine hexoxide, also known as chlorine dioxide, is a chemical compound with the formula Cl2O6. It is a powerful oxidizing agent and has various industrial and scientific applications. Let's dive into some fascinating facts about this compound.
Chemical Properties of Dichlorine Hexoxide
Understanding the chemical properties of dichlorine hexoxide can help us appreciate its uses and potential hazards.
- Dichlorine hexoxide is a yellowish-green gas at room temperature.
- It has a molecular weight of 166.90 g/mol.
- The compound is highly soluble in water, forming a pale yellow solution.
- It is a strong oxidizing agent, making it useful in various chemical reactions.
- Dichlorine hexoxide is unstable and can decompose explosively under certain conditions.
Uses of Dichlorine Hexoxide
This compound has several practical applications due to its strong oxidizing properties.
- It is used as a disinfectant in water treatment plants.
- Dichlorine hexoxide is employed in the bleaching of wood pulp in the paper industry.
- It serves as a biocide in the food industry to sanitize equipment and surfaces.
- The compound is used in oil and gas industries to control microbial growth.
- It is also utilized in textile manufacturing for bleaching fabrics.
Safety and Handling of Dichlorine Hexoxide
Due to its reactive nature, proper safety measures are crucial when handling dichlorine hexoxide.
- Dichlorine hexoxide is toxic and can cause respiratory issues if inhaled.
- It is a corrosive substance and can damage skin and eyes upon contact.
- The compound should be stored in cool, dry places away from direct sunlight.
- Proper ventilation is necessary when working with dichlorine hexoxide to avoid inhalation.
- Personal protective equipment (PPE) such as gloves and goggles should be worn when handling the compound.
Environmental Impact of Dichlorine Hexoxide
The environmental implications of using dichlorine hexoxide are significant and should be considered.
- Dichlorine hexoxide can react with organic matter in water, forming harmful by-products.
- It is known to be toxic to aquatic life, affecting fish and other organisms.
- The compound can contribute to air pollution if released in large quantities.
- Proper disposal methods are essential to prevent environmental contamination.
- Regulatory agencies monitor and control the use of dichlorine hexoxide to minimize its environmental impact.
Interesting Facts about Dichlorine Hexoxide
Here are some lesser-known yet intriguing facts about this compound.
- Dichlorine hexoxide was first synthesized in 1811 by Sir Humphry Davy.
- It has a distinctive odor similar to that of chlorine.
- The compound can be used in emergency situations to disinfect drinking water.
- Dichlorine hexoxide is sometimes used in wastewater treatment to remove contaminants.
- It has been studied for its potential use in medical applications, such as wound disinfection.
Dichlorine Hexoxide in Research
Ongoing research continues to uncover new uses and properties of dichlorine hexoxide.
- Scientists are exploring its use in antimicrobial coatings for medical devices.
- Research is being conducted on its potential to degrade pollutants in the environment.
- Studies are investigating its effectiveness in killing antibiotic-resistant bacteria.
- Dichlorine hexoxide is being tested for its ability to neutralize chemical warfare agents.
- Researchers are examining its role in advanced oxidation processes for water purification.
Final Thoughts on Dichlorine Hexoxide
Dichlorine hexoxide, a fascinating compound, holds a unique place in chemistry. Known for its strong oxidizing properties, it plays a crucial role in various industrial applications. This compound, with its distinct yellow-orange color, is highly reactive and must be handled with care. Its ability to decompose into chlorine and oxygen makes it both useful and hazardous. Despite its dangers, dichlorine hexoxide's applications in organic synthesis and as an oxidizing agent are invaluable. Understanding its properties and uses can help in harnessing its potential while ensuring safety. Whether you're a chemistry enthusiast or a professional, knowing these facts about dichlorine hexoxide can deepen your appreciation for this remarkable compound. Stay curious, stay safe, and keep exploring the wonders of chemistry.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
