When it comes to understanding the intricate world of chemistry, molecular orbitals play a crucial role. Among these, the sigma molecular orbital stands out as one of the most captivating subjects to explore. So, what exactly is a sigma molecular orbital? In simple terms, it is a type of molecular orbital that is formed by overlap of atomic orbitals along the internuclear axis. But there’s so much more to it than meets the eye!
In this article, we will delve into the fascinating world of sigma molecular orbitals and uncover 16 captivating facts that will leave you amazed. From understanding the concept behind sigma bonds to exploring their unique properties and applications, get ready to take a deep dive into the realm of molecular orbitals.
Key Takeaways:
- Sigma molecular orbital is like the glue that holds atoms together in molecules, shaping their properties and behavior. It’s super important in chemistry and helps us understand how things bond and react!
- Understanding sigma bonds helps scientists predict how chemicals will react and even create new materials with special properties, like super strong or extra conductive. It’s like a secret code for making cool stuff!
Sigma Molecular Orbital – The Building Block
Sigma molecular orbital is a fundamental concept in chemistry that forms the foundation for understanding the bonding and structure of molecules. It plays a crucial role in shaping the properties and behavior of chemical compounds.
The Overlapping Orbitals
Sigma molecular orbital is formed when two atomic orbitals, with the same or similar energies, overlap head-on. This overlap results in the formation of a bonding orbital with a high electron density in the region between the two nuclei.
Strong Bonding Character
The sigma molecular orbital is characterized by strong bonding interactions between the two atoms involved. This type of bonding is responsible for holding atoms together in molecules and contributes to the stability of chemical compounds.
Single Bonds and Sigma Bonds
In organic chemistry, sigma molecular orbitals are commonly associated with single bonds. These single bonds, also known as sigma bonds, are formed by the overlap of atomic orbitals along the bonding axis between two atoms.
Hybrid Orbitals and Sigma Bonds
Hybrid orbitals, formed by the mixing of atomic orbitals, are often involved in the formation of sigma bonds. The overlap of hybrid orbitals contributes to the strength and stability of sigma bonds in molecules.
Molecular Orbital Diagrams
Sigma molecular orbitals are represented in molecular orbital diagrams, which visually depict the energy levels and electron occupancy of the molecular orbitals in a compound. These diagrams provide valuable insights into the bonding and electronic structure of molecules.
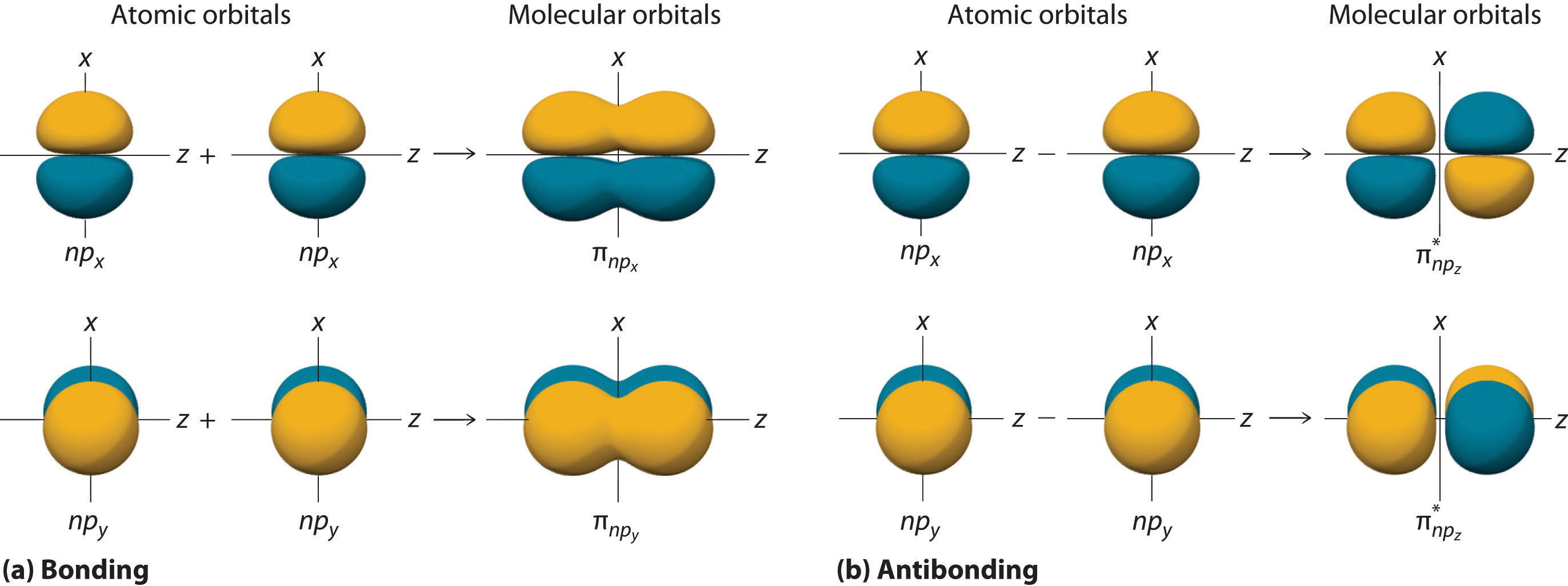
Pi Bonds and Sigma Bonds
Sigma bonds are often accompanied by pi bonds in chemical compounds. While sigma bonds result from the head-on overlap of atomic orbitals, pi bonds are formed by the sideways overlap of p-orbitals. The combination of sigma and pi bonds determines the overall bonding in molecules.
Delocalized Sigma Bonds
In some cases, sigma bonds can extend over multiple atoms, leading to delocalized bonding. This delocalization of electrons creates stability and gives rise to unique properties observed in certain compounds.
Role in Organic Chemistry
Sigma molecular orbitals are central to the study of organic chemistry. They are vital for understanding the structure, reactivity, and functional groups present in organic compounds.
Molecular Shape and Sigma Bonds
The formation of sigma bonds influences the molecular shape of compounds. The arrangement and orientation of sigma bonds determine the overall geometry of molecules, affecting their physical and chemical properties.
Sigma Bonds in Multiple Bonds
While sigma bonds are typically associated with single bonds, they can also occur in multiple bonds like double and triple bonds. In these cases, the sigma bond is the primary bond holding the atoms together.
Electron Density Distribution
Sigma molecular orbitals have a high electron density in the region between the nuclei of bonded atoms. This concentrated electron distribution stabilizes the molecule and contributes to its overall structure.
Molecular Stability and Sigma Bonds
The presence of sigma bonds promotes molecular stability by allowing for efficient sharing of electrons between atoms. This stability is crucial for the existence and functionality of chemical compounds.
Molecular Orbital Theory
Sigma molecular orbital is a cornerstone of the molecular orbital theory, which provides a comprehensive framework for understanding the electronic structure and bonding in molecules.
Significance in Chemical Reactions
The formation and breaking of sigma bonds play a vital role in chemical reactions. Understanding the behavior of sigma bonds is essential for predicting and studying the reactivity of compounds.
Applications in Materials Science
The knowledge and manipulation of sigma molecular orbitals have significant applications in materials science. This understanding contributes to developing new materials with tailored properties, such as improved conductivity or enhanced strength.
In conclusion, the sigma molecular orbital is a fascinating concept in chemistry, serving as the building block for understanding molecular bonding and structure. Its significance spans across various branches of chemistry and has profound implications in fields such as organic chemistry, materials science, and chemical reactions. Exploring the nature and properties of sigma bonds opens up avenues for further advancements and discoveries in the realm of chemical compounds.
Conclusion
In conclusion, sigma molecular orbitals are a fascinating aspect of chemistry that play a crucial role in explaining the bonding and structure of molecules. Understanding sigma molecular orbitals allows scientists to predict and explain chemical reactions, determine the stability of compounds, and explore the electronic properties of molecules.
Throughout this article, we have delved into 16 captivating facts about sigma molecular orbitals. From their formation through the overlap of atomic orbitals to their role in determining the strength of chemical bonds, sigma orbitals offer valuable insights into the world of chemistry.
By exploring the concept of sigma molecular orbitals, we can deepen our understanding of the intricate nature of chemical bonding and the behavior of molecules in various chemical reactions. As research in this field continues, we can expect even more fascinating discoveries to unfold, pushing the boundaries of our knowledge and revolutionizing the field of chemistry.
FAQs
1. What is a sigma molecular orbital?
A sigma molecular orbital is a type of molecular orbital formed by the overlap of atomic orbitals in a molecule. It is responsible for the primary bonding between atoms.
2. How are sigma molecular orbitals formed?
Sigma molecular orbitals are formed by the linear combination of atomic orbitals that have proper symmetry and energy compatibility to create a bonding interaction.
3. What is the significance of sigma molecular orbitals?
Sigma molecular orbitals play a crucial role in determining the strength of chemical bonds, the stability of compounds, and the electronic properties of molecules.
4. What is the difference between sigma and pi molecular orbitals?
Sigma orbitals result from head-to-head overlap of atomic orbitals, while pi orbitals result from sideways overlap. Sigma bonds are characterized by electron density along the internuclear axis, while pi bonds have electron density above and below the internuclear axis.
5. Can sigma molecular orbitals delocalize?
While sigma molecular orbitals primarily form localized bonds, in some cases, they can delocalize to spread their electron density over a larger region.
6. How do sigma molecular orbitals influence chemical reactions?
Sigma molecular orbitals determine the energetics and reactivity of chemical reactions. They dictate the stability of reactants and products and influence the activation energy required for a chemical change.
7. Are sigma molecular orbitals present in all molecules?
Sigma molecular orbitals are present in all molecules since they are responsible for the primary bonding between atoms. However, the types of sigma orbitals and their relative energies can vary depending on the nature of the atoms involved.
Exploring sigma molecular orbitals is just the beginning of understanding chemical bonding. Dive deeper into valence bond theory to grasp how atoms share electrons. Chemical bonds form the foundation of all matter, from simple molecules to complex structures. Discover how orbital hybridization plays a crucial role in determining molecular geometry and reactivity. Continue your journey through chemistry and unravel the secrets behind the fascinating world of chemical interactions.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
