The empirical formula is one of the fundamental concepts in chemistry that helps us understand the composition of compounds. It provides a concise way to represent the simplest ratio of atoms present in a chemical compound. By expressing the empirical formula, chemists can gain valuable insights into the molecular structure and properties of substances.
In this article, we will delve into the world of empirical formulas and explore some fascinating facts that will leave you astounded. From its historical significance to its practical applications, the empirical formula plays a crucial role in understanding the building blocks of matter. So, let’s embark on this exciting journey and uncover 15 astounding facts about the empirical formula.
Key Takeaways:
- The empirical formula shows the simplest ratio of elements in a compound, helping scientists understand its basic composition and properties.
- Empirical formulas play a crucial role in stoichiometry calculations and can be used to predict chemical reactions, making them essential in chemistry research and discovery.
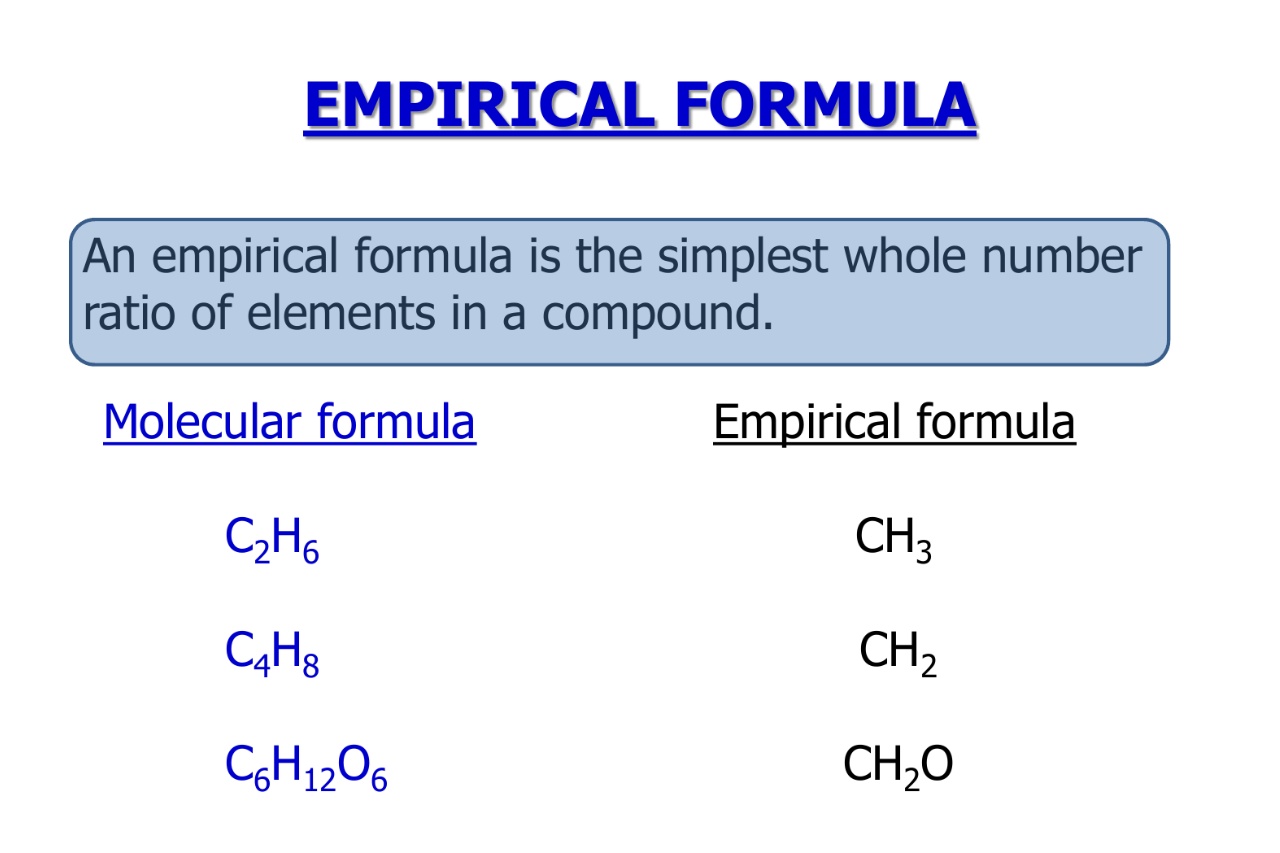
The empirical formula represents the simplest ratio of elements in a compound.
The empirical formula provides valuable information about the composition of a compound, showing the relative number of atoms of each element present.
It can be determined through experimental data.
By analyzing the masses or percentages of elements in a compound, scientists can calculate the empirical formula.
The empirical formula is not always the same as the molecular formula.
While the empirical formula represents the simplified ratio of elements, the molecular formula provides the actual number of atoms in a compound.
Empirical formulas are widely used in stoichiometry calculations.
Stoichiometry involves determining the quantitative relationships between reactants and products in a chemical reaction, and empirical formulas play a crucial role in these calculations.
The empirical formula is essential in determining the formula mass of a compound.
By knowing the empirical formula and the atomic masses of the elements, scientists can calculate the formula mass, which is important for various calculations in chemistry.
The empirical formula can be the same for different compounds.
Different compounds can have the same empirical formula if they have the same elemental composition but different molecular structures.
Empirical formulas are particularly useful in organic chemistry.
Organic compounds often have complex molecular formulas, but determining their empirical formulas can provide valuable insight into their chemical properties and behavior.
The empirical formula plays a crucial role in combustion analysis.
Combustion analysis is a technique used to determine the empirical formula of an unknown compound by burning it and analyzing the resulting products.
Empirical formulas can be represented using subscripts or parentheses.
A subscript is used to indicate the number of atoms of each element, while parentheses are used to group atoms together when there is more than one of a particular element.
Empirical formulas provide a simplified representation of complex compounds.
By reducing a compound to its empirical formula, scientists can better understand its basic composition and properties.
The empirical formula is the basis for determining the percent composition of a compound.
The percent composition shows the percentage by mass of each individual element in a compound based on its empirical formula.
Empirical formulas can be used to predict chemical reactions.
By knowing the empirical formulas of reactants and products, chemists can predict the outcome of a chemical reaction and design new compounds.
The empirical formula is used to determine the limiting reactant in a chemical reaction.
Knowing the empirical formulas allows scientists to calculate the stoichiometric ratios and determine which reactant will be fully consumed.
The empirical formula is a powerful tool in organic synthesis.
By using the empirical formula of a compound, organic chemists can design and create new molecules with specific properties and functions.
The empirical formula can provide insight into molecular symmetry.
The arrangement of atoms in a molecule can be deduced from the empirical formula, helping scientists understand its symmetry and overall structure.
Conclusion
In conclusion, empirical formulas play a crucial role in the field of chemistry. They provide us with essential information about the relative proportions of different elements in a compound. By determining the empirical formula, scientists can gain insights into the fundamental building blocks of molecules, enabling them to better understand their properties and behaviors.By using empirical formulas, chemists can make predictions about the composition and behavior of compounds, laying the foundation for further research and experimentation. This knowledge is invaluable in various areas of chemistry, ranging from pharmaceuticals to materials science.Understanding empirical formulas unlocks a deeper understanding of the world around us at a molecular level. Through precise measurements and calculations, scientists have been able to uncover astounding facts about empirical formulas, revealing the beauty and complexity of the chemical world.So, next time you come across an empirical formula, take a moment to appreciate the wealth of information it holds and the role it plays in advancing our knowledge of chemistry.
FAQs
1. What is an empirical formula?
An empirical formula is the simplest ratio of elements in a compound, representing the relative number of atoms of each element present.
2. How is the empirical formula determined?
The empirical formula is determined by finding the ratio of the number of moles of each element in a compound. This is usually done by analyzing the mass of each element in a sample.
3. Can an empirical formula be the same as a molecular formula?
Yes, an empirical formula can be the same as a molecular formula if the compound consists of a single molecule.
4. What is the significance of empirical formulas?
Empirical formulas provide valuable information about the relative proportions of elements in a compound. This knowledge helps chemists understand the composition and behavior of substances, leading to advancements in various fields.
5. How do empirical formulas help in chemical reactions?
Empirical formulas help chemists determine the starting materials and products involved in a chemical reaction, allowing for accurate stoichiometric calculations.
6. Can empirical formulas be changed?
No, the empirical formula of a compound remains constant regardless of the scale or conditions under which the compound is measured.
7. Are empirical formulas used in everyday life?
Absolutely! Empirical formulas are used in industries such as pharmaceuticals, food production, and materials science, aiding in the development of new products and technologies that improve our everyday lives.
8. Can empirical formulas predict the properties of a compound?
While empirical formulas provide information about the relative proportions of elements, they do not provide specific details about the properties of individual compounds. Additional experimentation and analysis are needed to fully understand a substance’s properties.
Unraveling empirical formulas is just the beginning of your journey into the fascinating world of chemistry. Dive deeper into the captivating realm of chemistry and explore its countless wonders. Master the art of stoichiometry and unlock the secrets behind chemical reactions. Discover the intricacies of molecular formulas and how they shape the compounds that surround us. Embark on a thrilling adventure through the pages of these articles and expand your knowledge of the chemical universe.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
