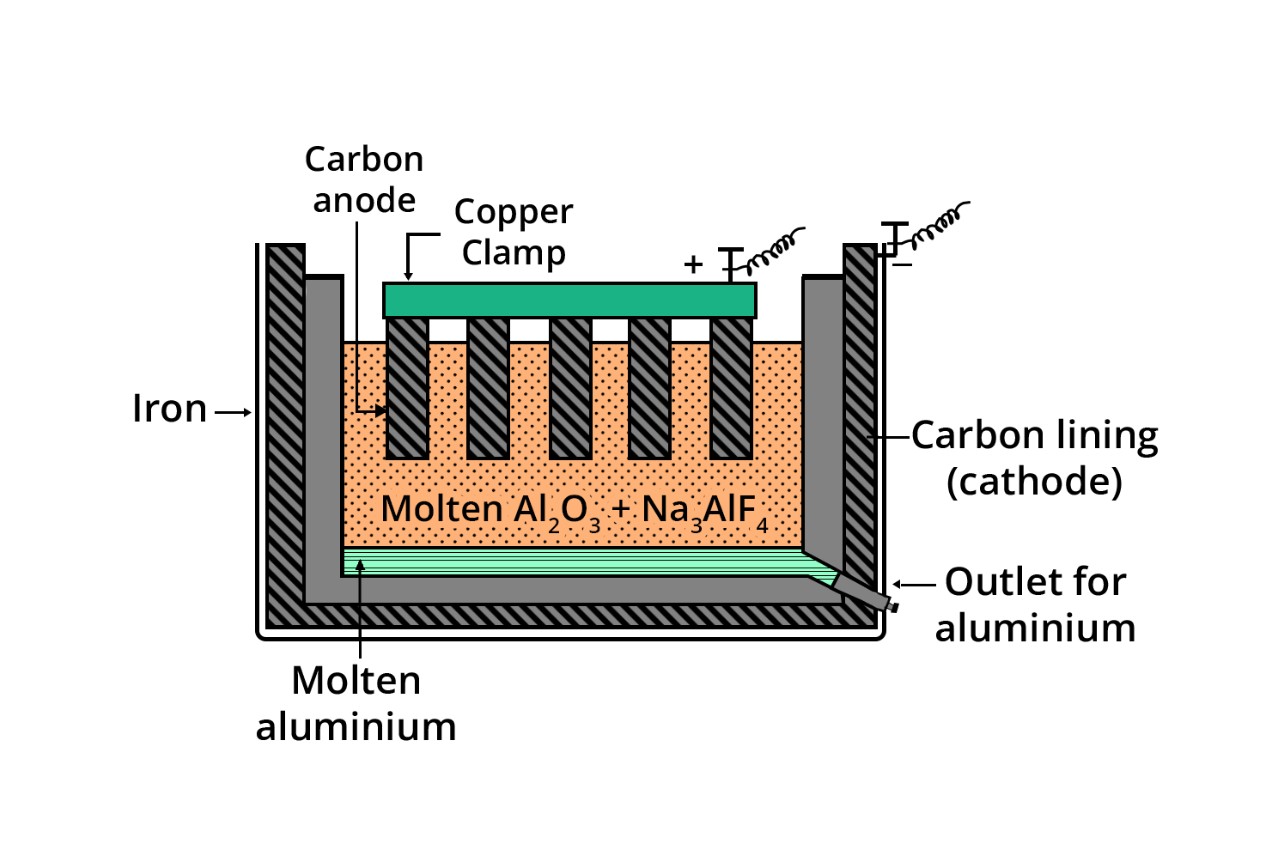
The Hall-Heroult process is a groundbreaking method used in the extraction of pure aluminum, revolutionizing the production of this versatile metal. Developed by Charles Martin Hall and Paul Héroult independently in the late 19th century, this process has become essential in the aluminum industry. It involves electrolysis of alumina, the oxide of aluminum, to obtain the elemental metal.
In this article, we will delve into the fascinating world of the Hall-Heroult process and uncover 13 astounding facts about it. From the historical significance of its invention to the intricate steps involved in the process, we will explore the science and innovation behind this method. Get ready to be amazed by the ingenuity and impact of the Hall-Heroult process on the modern world.
Key Takeaways:
- The Hall-Heroult process is a super cool way to make aluminum and it’s used to make most of the aluminum in the world. It’s like a magic trick that turns alumina into shiny, useful metal!
- Even though the Hall-Heroult process needs a lot of energy and makes carbon dioxide, it’s still the best way to make top-quality aluminum. And it can even recycle old aluminum to save energy!
The Hall-Heroult process revolutionized aluminum production
The Hall-Heroult process is a groundbreaking technique that made the mass production of aluminum economically viable. Prior to this, aluminum was a precious metal and more valuable than gold due to its scarcity.
It is used to produce the majority of the world’s aluminum
The Hall-Heroult process is used to produce over 90% of the world’s aluminum. This process plays a pivotal role in meeting the global demand for this versatile metal.
The process involves the electrolysis of alumina
In the Hall-Heroult process, alumina (Al2O3) is dissolved in a molten cryolite bath and subjected to electrolysis. This electrolytic process allows for the extraction of metallic aluminum from the alumina compound.
Cryolite is used as a solvent for alumina
Cryolite, a mineral compound consisting of sodium, aluminum, and fluoride, is used as a solvent to lower the melting point of alumina. This allows for the efficient dissolution and electrolysis of alumina in the Hall-Heroult process.
The process requires significant electrical energy
The Hall-Heroult process is highly energy-intensive. It requires massive amounts of electrical energy to sustain the electrolytic reaction and produce aluminum. The cost of electricity is a major factor in the overall production cost of aluminum.
The process operates at high temperatures
The Hall-Heroult process operates at temperatures of around 950-980 degrees Celsius. The high temperatures are necessary to maintain the molten cryolite bath and facilitate the electrolysis of alumina.
It produces high-quality aluminum
The Hall-Heroult process yields high-purity aluminum with a purity level of over 99%. This makes it suitable for a wide range of applications, including aerospace, construction, and automotive industries.
It generates large amounts of carbon dioxide
One of the challenges associated with the Hall-Heroult process is the generation of carbon dioxide (CO2) emissions. The carbon anodes used in the process release CO2 during the smelting of alumina.
The process creates valuable byproducts
Although the Hall-Heroult process primarily aims to produce aluminum, it also generates valuable byproducts. These include carbon/graphite materials, fluorine compounds, and various alloys that have industrial applications.
It requires regular maintenance and replacement of components
The Hall-Heroult process involves the use of graphite electrodes, which degrade over time due to the extreme conditions they are subjected to. Regular maintenance and replacement of these electrodes are necessary to ensure efficient aluminum production.
Recycling of aluminum saves energy and resources
The Hall-Heroult process can also be used to recycle aluminum. Recycling aluminum requires significantly less energy compared to primary production, making it an environmentally friendly option that conserves resources.
The process has evolved with technological advancements
Since its inception, the Hall-Heroult process has undergone significant advancements with the advent of new technologies. These advancements aim to improve energy efficiency, reduce emissions, and enhance overall productivity.
The Hall-Heroult process continues to dominate aluminum production
Despite the availability of alternative methods, the Hall-Heroult process remains the most widely used technique for aluminum production. Its efficiency, cost-effectiveness, and ability to produce high-quality aluminum have solidified its position in the industry.
Conclusion
The Hall-Heroult process is an astounding method for producing aluminum on a large scale. Its invention revolutionized the aluminum industry, providing a cost-effective and efficient way to extract this versatile metal. Through the use of a carbon anode and a molten electrolyte, this process allows for the separation of aluminum from its oxide compound, resulting in the production of pure aluminum. The Hall-Heroult process has greatly contributed to the modern world, enabling the development of lightweight and durable aluminum products that are used in various industries such as aerospace, automotive, and construction. Its significance cannot be overstated, as it continues to shape our everyday lives.
FAQs
Q: What is the Hall-Heroult process?
A: The Hall-Heroult process is a method used to produce aluminum by electrolysis. It involves the separation of aluminum from its oxide compound by passing an electric current through a molten electrolyte containing alumina.
Q: Who invented the Hall-Heroult process?
A: The Hall-Heroult process was jointly developed by Charles Martin Hall and Paul Héroult in the late 19th century. Their groundbreaking work resulted in the commercialization of aluminum production.
Q: What is the significance of the Hall-Heroult process?
A: The Hall-Heroult process revolutionized the aluminum industry by providing a cost-effective and efficient means of producing aluminum on a large scale. It enabled the widespread use of aluminum in various industries, thanks to its lightweight, durability, and corrosion-resistant properties.
Q: What are some applications of aluminum produced through the Hall-Heroult process?
A: Aluminum produced through the Hall-Heroult process finds applications in numerous industries, including aerospace, automotive, construction, packaging, and electrical. It is used in the production of aircraft, cars, buildings, beverage cans, electrical wiring, and more.
Q: Are there any environmental concerns associated with the Hall-Heroult process?
A: The Hall-Heroult process consumes a significant amount of energy, mainly due to the high temperature required to melt alumina and the electrolysis process. The energy-intensive nature of the process contributes to greenhouse gas emissions. However, efforts are being made to improve energy efficiency and reduce the environmental impact associated with aluminum production.
The Hall-Heroult process is just one of many fascinating industrial processes that shape our modern world. Curious minds might also find interest in exploring the principles of electrolysis, which underlies numerous chemical reactions and industrial applications. For those drawn to the wider world of industrial chemistry, the Ostwald process offers surprising insights into the production of essential compounds. And no discussion of industrial processes would be complete without delving into the rich history and ongoing innovations in the field of metallurgy, where ancient techniques meet cutting-edge science to create the materials that build our future.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
