Freezing point depression is a fascinating phenomenon in the field of chemistry that occurs when the freezing point of a liquid is lowered due to the presence of a solute. This intriguing effect has many practical applications and plays a crucial role in various industries, including medicine, food preservation, and materials science. To fully appreciate the significance of freezing point depression, it is essential to delve into some extraordinary facts surrounding this phenomenon. In this article, we will explore 12 intriguing facts about freezing point depression, shedding light on its underlying principles, real-world implications, and even some mind-boggling examples. So, if you’re ready to be captivated by the wonders of chemistry, let’s dive into the world of freezing point depression!
Key Takeaways:
The phenomenon of Freezing Point Depression
Freezing Point Depression is a fascinating phenomenon in chemistry that occurs when the freezing point of a solvent is lowered by adding a solute to it. It is a crucial concept in various fields, including biochemistry, pharmaceuticals, and environmental science. Let’s delve into 12 extraordinary facts about Freezing Point Depression that will expand our understanding of this intriguing phenomenon.
The Colligative Property of Freezing Point Depression
One of the key factors contributing to Freezing Point Depression is the colligative property. This means that the extent of Freezing Point Depression depends solely on the number of solute particles present, rather than their nature.
Vapor Pressure Lowering
Freezing Point Depression occurs because the solute particles disrupt the crystal lattice formation of the solvent, reducing its vapor pressure. Consequently, the solvent requires a lower temperature to reach its freezing point.
Molal Freezing Point Depression Constant
Each solvent has its own unique Molal Freezing Point Depression Constant (Kf). This constant is a measure of how much the freezing point of the solvent decreases when a solute is added.
Depression of Freezing Point in Solutions
When a non-volatile solute dissolves in a solvent, it lowers the freezing point of the resulting solution. The greater the concentration of the solute, the larger the freezing point depression observed.
Relation to Osmotic Pressure
Freezing Point Depression is closely related to osmotic pressure. Osmosis occurs when there is a difference in solute concentration across a semipermeable membrane, leading to the movement of solvent molecules to balance the concentrations.
Cryoscopic Constant
The Cryoscopic Constant (Kc) is another term used to describe the extent of Freezing Point Depression in a specific solvent. It is directly proportional to the molal concentration of the solute.
Applications in Antifreeze Solutions
Freezing Point Depression finds practical applications in the creation of antifreeze solutions. By adding substances like ethylene glycol to water, the freezing point of the solution is depressed, allowing it to remain liquid even at low temperatures.
Importance in Food Preservation
Freezing Point Depression plays a vital role in food preservation, as it prevents the formation of large ice crystals that can damage the food’s texture and quality. Adding salt to an ice bath, for example, lowers the melting point of the ice, enabling better preservation of perishable items.
Freezing Point Depression and Molecular Weight Determination
Scientists can utilize Freezing Point Depression to determine the molecular weight of unknown substances. By measuring the extent of Freezing Point Depression, they can calculate the number of solute particles present and infer the molecular weight.
Dependence on Solute-Solvent Interactions
The extent of Freezing Point Depression can vary depending on the nature of the solute-solvent interactions. For example, when the solute and solvent have strong interactions, the freezing point depression is more pronounced.
Law of Thermodynamics
Freezing Point Depression follows the laws of thermodynamics. It is a result of the decrease in the system’s Gibbs free energy, indicating a more stable state of the solution compared to the pure solvent.
Conclusion
In conclusion, the phenomenon of freezing point depression is a fascinating concept that has numerous applications in various fields of science and technology. By understanding how solute particles affect the freezing point of a solvent, scientists and researchers can develop new materials, improve processes, and enhance the performance of a wide range of products.
From the creation of antifreeze solutions to the production of cryogenic materials, the study of freezing point depression has paved the way for groundbreaking discoveries and advancements. By harnessing this scientific principle, we can unlock a world of possibilities and explore new frontiers in fields such as chemistry, medicine, and engineering.
So, the next time you come across freezing point depression, remember the extraordinary facts we’ve discussed and appreciate the profound impact this phenomenon has on our daily lives and the world around us.
FAQs
1. What is freezing point depression?
Freezing point depression is the phenomenon where the freezing point of a solvent is lowered when a solute is added to it.
2. How does freezing point depression occur?
When a solute is added to a solvent, it disrupts the regular crystal lattice structure of the solvent, making it more difficult for the solvent molecules to arrange themselves in an orderly manner during freezing, resulting in a lower freezing point.
3. What are some practical applications of freezing point depression?
Freezing point depression is used in the production of antifreeze solutions, preservation of food through freezing, cryopreservation of biological materials, and various industrial processes that require low-temperature environments.
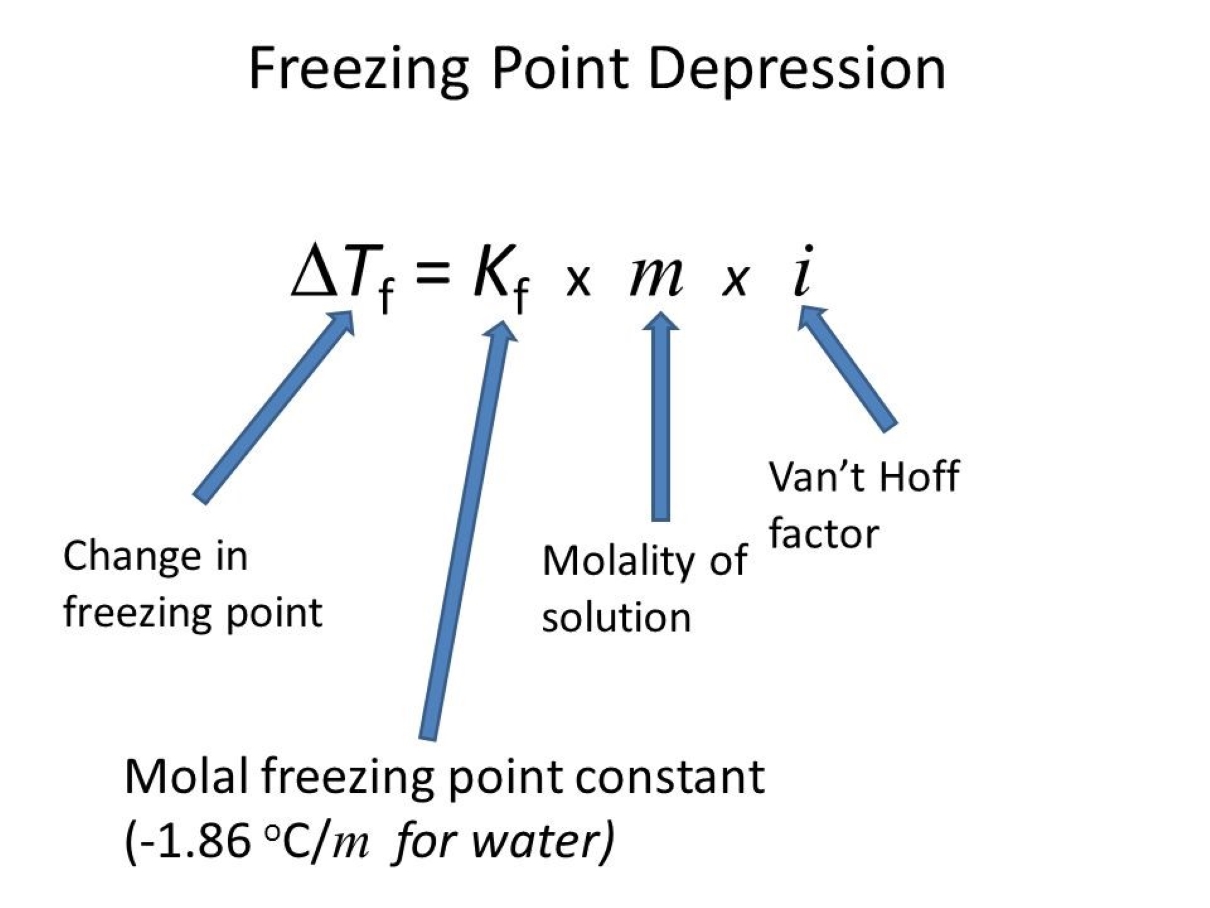
4. How is freezing point depression calculated?
The freezing point depression can be calculated using the equation: ?T = Kf * m * i, where ?T is the change in freezing point, Kf is the cryoscopic constant, m is the molality of the solute, and i is the van’t Hoff factor.
5. Are there any limitations to freezing point depression?
Although freezing point depression is a valuable phenomenon, it relies on ideal conditions and assumes ideal solutions. Deviations from ideal behavior can affect the accuracy of calculations and predictions.
Freezing point depression's fascinating properties make solutions resist freezing, enabling antifreeze to protect engines and allowing molecular weight determination. This phenomenon also helps preserve food by lowering freezing points. Exploring molality provides deeper insights into solute concentration's effects on colligative properties like freezing point depression. Understanding these principles unlocks a world of practical applications and scientific understanding.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
