Osmosis is a fascinating natural process that occurs in various living organisms as well as in our everyday lives. It is a fundamental concept in the field of chemistry and biology, and it plays a crucial role in maintaining the balance of fluids within our bodies. Understanding the concept of osmosis can help us comprehend how substances move across cell membranes and how the body regulates hydration.
In this article, we will delve deeper into the topic of osmosis and uncover some captivating facts about this intriguing phenomenon. From its discovery by Jean-Antoine Nollet to its applications in everyday life, you will learn about the importance of osmosis in various scientific fields and how it impacts our physical well-being. So, let’s dive in and explore 11 captivating facts that will expand your knowledge of osmosis.
Key Takeaways:
- Osmosis is the movement of water molecules from an area of low solute concentration to an area of high solute concentration, crucial for maintaining balance in living organisms and water purification processes.
- Osmosis is like a natural balancing act, helping plants stay upright, allowing our bodies to rehydrate, and even assisting in waste removal. It’s a fascinating process that keeps the world in harmony!
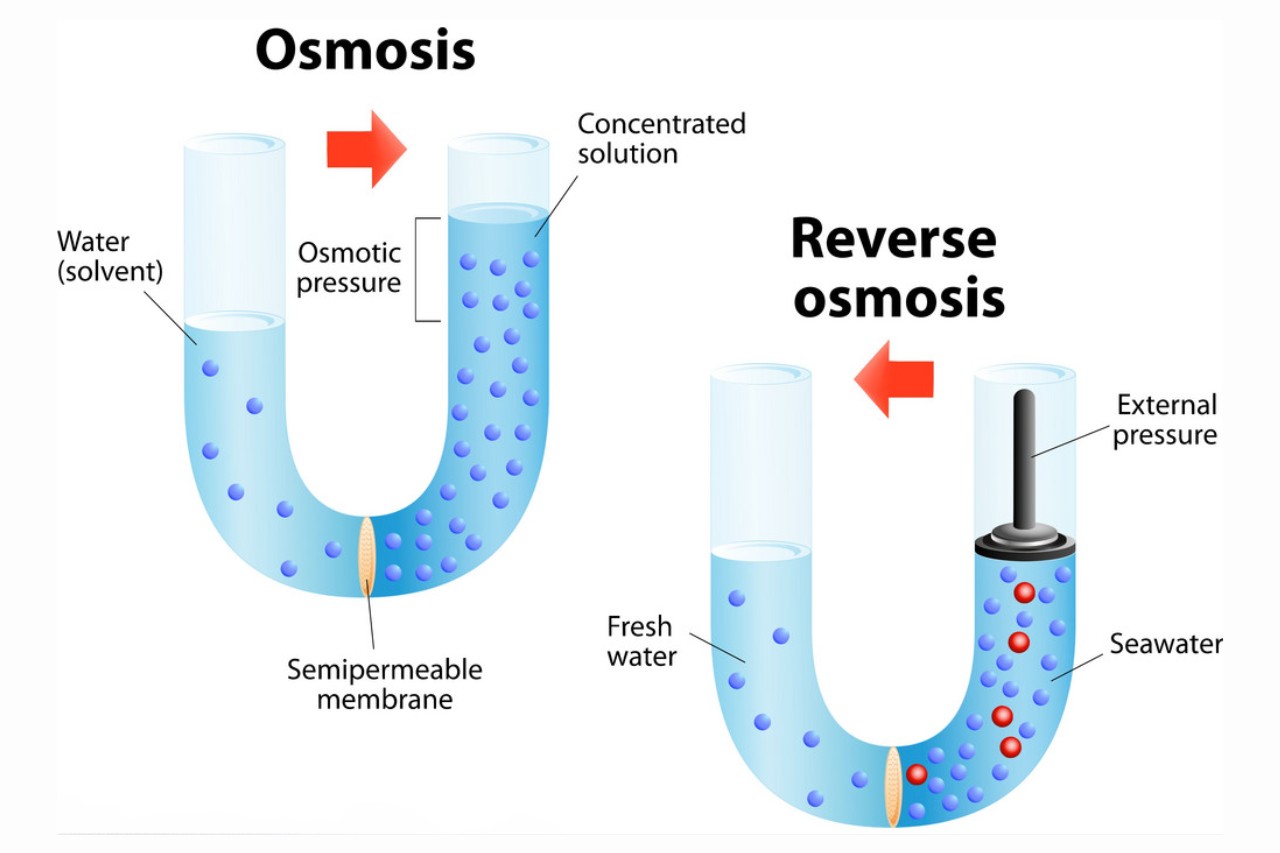
Osmosis is the movement of solvent molecules from an area of low solute concentration to an area of high solute concentration through a semipermeable membrane.
Osmosis is a vital process in biological systems, playing a crucial role in maintaining the balance of water and solutes within cells and organisms.
Osmosis is driven by the concentration gradient between the two solutions.
When there is a difference in solute concentration across a semipermeable membrane, water molecules will move across the membrane in an attempt to equalize the concentration on both sides.
Osmosis is responsible for the process of rehydration.
When we consume drinks that have a higher water concentration than our body fluids, osmosis helps in replenishing the lost water by moving it from the digestive system into the bloodstream.
Osmosis is essential for plant cells to maintain turgidity.
When plant cells are in a hypotonic solution (lower solute concentration outside the cell), water moves in through osmosis, causing the cell to swell and become turgid. This turgidity maintains the structural integrity of the plant.
Reverse osmosis is a process used for water purification.
In reverse osmosis, pressure is applied to a solution with higher solute concentration, forcing the solvent (usually water) through a semipermeable membrane, leaving behind the impurities and producing pure water.
Osmosis is responsible for the uptake of water and nutrients in plant roots.
Plant roots have specialized structures, such as root hairs, that increase the surface area for water absorption. Osmosis facilitates the movement of water and dissolved nutrients into the root cells.
Osmosis can cause red blood cells to shrink or swell.
When red blood cells are exposed to a hypotonic solution, water enters the cells through osmosis, causing them to swell and potentially burst. Conversely, in a hypertonic solution, water leaves the cells, leading to shrinkage.
Osmosis can be affected by external factors such as temperature and pressure.
Higher temperatures generally increase the rate of osmosis, as molecules gain more kinetic energy. Increased pressure can also alter the rate at which osmosis occurs.
Osmosis allows for waste removal in living organisms.
Osmosis helps in the removal of waste products from cells and tissues. The movement of water from an area of low solute concentration to an area of high solute concentration aids in eliminating waste through osmotic pressure.
Osmotic pressure is the pressure required to prevent osmosis from occurring.
This pressure is proportional to the concentration difference between the two solutions. It is an essential concept in understanding osmosis and its effects on various systems.
Osmosis is not limited to water.
While osmosis is commonly associated with the movement of water molecules, it can also occur with other solvents, such as alcohols or gases, across a semipermeable membrane.
These fascinating facts about osmosis highlight the importance of this process in various biological and physical systems. Whether it’s in plant cells, water purification, or waste removal, osmosis plays a vital role in maintaining equilibrium and supporting life.
So, the next time you witness the effects of osmosis, think about the incredible journey of molecules through semipermeable membranes and appreciate the wonders of this natural phenomenon!
Conclusion
In conclusion, osmosis is an intriguing process that plays a vital role in various biological and chemical phenomena. It is the movement of solvent molecules from an area of higher concentration to an area of lower concentration through a semi-permeable membrane. Osmosis is not only essential for the survival of living organisms, but it also has numerous applications in industries such as pharmaceuticals, food processing, and water treatment.Understanding the principles of osmosis helps scientists and researchers develop innovative solutions for medical treatments, improve food preservation methods, and ensure access to clean drinking water. By studying osmosis, we can gain a deeper insight into the intricate mechanisms that govern the behavior of molecules and the functioning of cells.Overall, the captivating facts about osmosis reveal its significance and complexity in the world of chemistry and biology. The discovery and exploration of osmosis continue to shape our understanding of the natural world and provide valuable insights into the microscopic processes that sustain life.
FAQs
1. What is osmosis?
Osmosis is the process by which solvent molecules move from an area of higher concentration to an area of lower concentration through a semi-permeable membrane.
2. How does osmosis differ from diffusion?
While both processes involve the movement of particles, diffusion refers to the movement of solute particles from an area of higher concentration to lower concentration, whereas osmosis specifically involves the movement of solvent molecules.
3. What is a semi-permeable membrane?
A semi-permeable membrane is a membrane that allows certain molecules or ions to pass through it while restricting the passage of others based on their size and charge.
4. What are some real-world examples of osmosis?
Examples of osmosis in everyday life include the absorption of water by plant roots, the process of osmosis in kidney function, and the preservation of fruits and vegetables by controlling the osmotic pressure in food packaging.
5. How is osmosis used in scientific research and industries?
Osmosis plays a crucial role in various fields, including medicine, biotechnology, and water treatment. It helps in drug delivery systems, tissue engineering, desalination of water, and purification processes.
Osmosis captivates with its essential role in life, from hydration to nutrient uptake. Dive deeper into osmosis's mesmerizing world by exploring mind-blowing facts that showcase its power and versatility. Uncover more fascinating insights about this process, including how it relates to the beloved cartoon character Osmosis Jones. Embark on a journey to quench your thirst for knowledge and appreciate osmosis's significance in our lives.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
