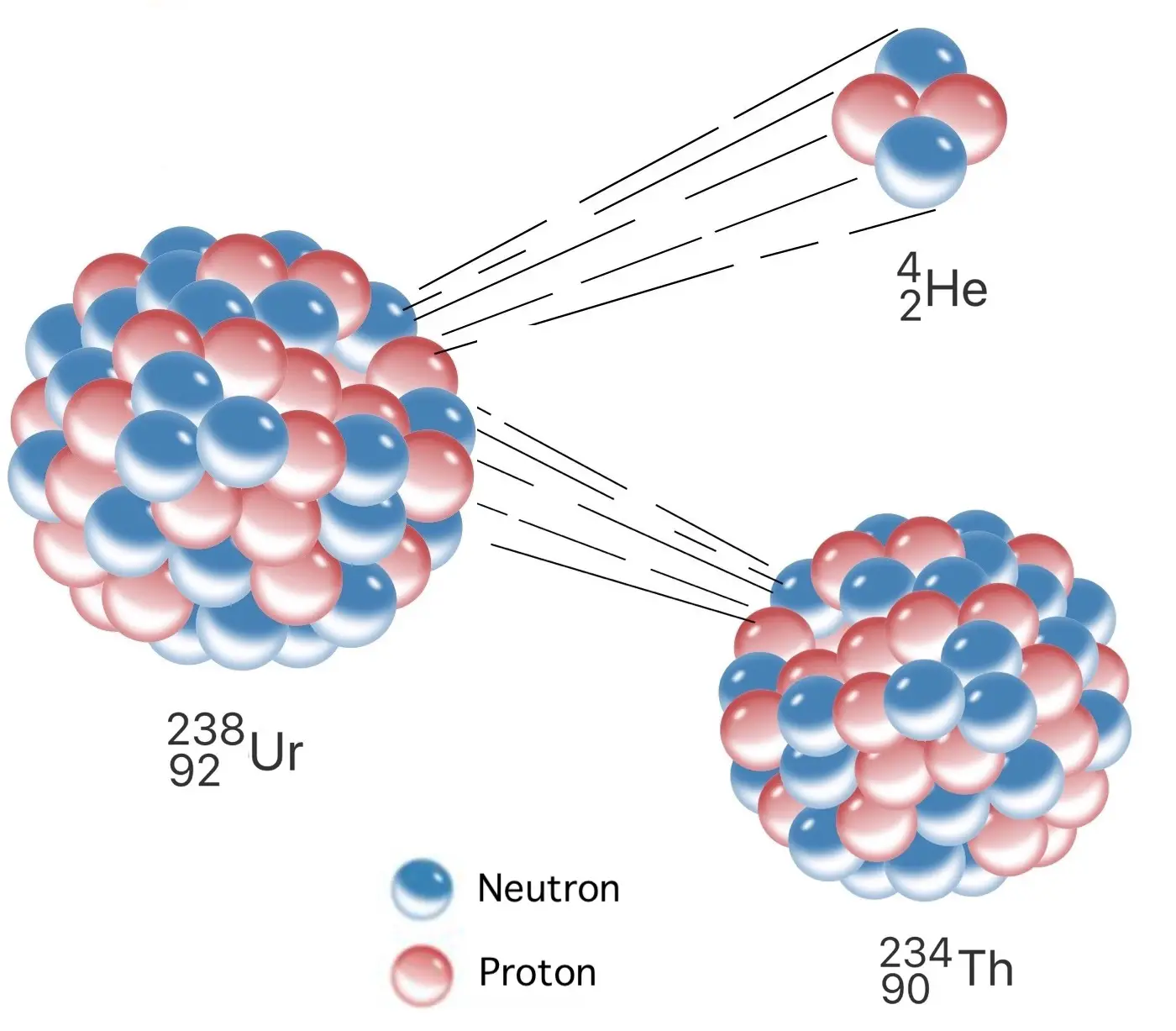
Alpha decay is a fascinating phenomenon in the world of physics. It occurs when an atomic nucleus releases an alpha particle, which is composed of two protons and two neutrons. This process is often associated with the emission of a high-energy helium nucleus. Alpha decay is one of the three major types of radioactive decay, along with beta decay and gamma decay. In alpha decay, the parent nucleus undergoes a transformation into a daughter nucleus with a lower atomic number and mass number. This unique process has several extraordinary facts associated with it that showcase the intricate nature of atomic behavior. In this article, we will delve into 13 extraordinary facts about alpha decay, shedding light on its significance and its implications in the field of physics.
Key Takeaways:
- Alpha decay is a natural process where heavy elements release alpha particles, helping them become more stable. It’s like a tiny atomic makeover!
- Alpha decay is super useful! It helps us understand the age of rocks, creates elements in stars, and even fights cancer through targeted therapy. It’s like a superhero in the atomic world!
Alpha decay is a type of radioactive decay process.
Alpha decay is a natural phenomenon in which an atomic nucleus emits an alpha particle. This decay process occurs in heavy and unstable elements as a means to achieve a more stable configuration.
Alpha particles are made up of two protons and two neutrons.
An alpha particle is essentially the same as a helium nucleus, consisting of two protons and two neutrons. It has a positive charge and is relatively large compared to other subatomic particles.
Alpha decay results in the reduction of the parent nucleus’s atomic number by two.
When an atomic nucleus undergoes alpha decay, it loses two protons, leading to a decrease in its atomic number. This transformation often creates a completely different element.
Alpha decay releases a significant amount of energy.
During the process of alpha decay, a considerable amount of energy is released in the form of kinetic energy carried by the emitted particle. This energy can be harnessed for various applications, including power generation.
Alpha particles have low penetrating power.
Alpha particles possess limited penetrating power due to their larger size and positive charge. They can be easily stopped by a sheet of paper or a few centimeters of air, making them less hazardous compared to other forms of radiation.
Alpha decay plays a crucial role in the formation of elements.
Alpha decay is responsible for the natural synthesis of heavier elements through a process known as nucleosynthesis. This phenomenon occurs in stars and helps in the production of elements such as uranium and radium.
The discovery of alpha particles paved the way for nuclear physics.
Ernest Rutherford’s famous experiments involving the scattering of alpha particles played a pivotal role in unraveling the structure of the atom and understanding the concept of atomic nucleus.
Alpha decay has a characteristic decay constant.
The rate at which alpha decay occurs in a radioactive element is determined by its characteristic decay constant. This decay constant is specific to each element and remains constant over time.
Alpha decay can be used in radiometric dating.
Thanks to the predictable nature of alpha decay, scientists can use it to determine the age of rocks and minerals through a process called radiometric dating. This technique has been instrumental in studying the Earth’s geological history.
The half-life of alpha decay varies for different isotopes.
Each isotope undergoing alpha decay has a unique half-life, which is the time it takes for half of the initial quantity to decay. Some isotopes have incredibly long half-lives, while others decay much more rapidly.
Alpha decay is classified as a spontaneous radioactive decay.
In spontaneous radioactive decay, the decay process occurs without any external influence. The emission of alpha particles is a self-driven process governed by the inherent instability of certain atomic nuclei.
Alpha decay contributes to nuclear radiation.
Alpha particles are a form of ionizing radiation, and their emission during alpha decay is a component of natural background radiation. Although they are less harmful externally, they can cause significant damage if inhaled or ingested.
Scientists can harness alpha decay for medical applications.
Alpha emitters, such as radium-223, are used in targeted alpha therapy to treat certain types of cancer. The high energy and short range of alpha particles are advantageous for precise tumor destruction.
The “13 Extraordinary Facts About Alpha Decay”
Alpha decay is a fascinating phenomenon that plays a vital role in understanding the behavior of atomic nuclei. With its unique properties and applications, alpha decay continues to captivate scientists and hold significant scientific and practical implications.
Conclusion
Alpha decay is a fascinating phenomenon in nuclear physics that involves the emission of an alpha particle from a radioactive nucleus. In this article, we explored 13 extraordinary facts about alpha decay, uncovering its significance and impact in the field of atomic research.
We discussed how alpha decay plays a crucial role in understanding the stability and decay of radioactive elements, as well as its implications in radiation therapy and nuclear energy. The remarkable properties of alpha particles, such as their high ionizing power and limited penetration depth, make them useful in various applications.
Additionally, we delved into the concept of half-life and how it relates to alpha decay, shedding light on the mathematical aspect of radioactive decay. The discovery and understanding of alpha decay have contributed extensively to our knowledge of the universe and paved the way for groundbreaking discoveries in nuclear physics.
Overall, alpha decay is a captivating phenomenon that continues to intrigue scientists and researchers as they unravel its mysteries and explore its potential applications.
FAQs
1. What is alpha decay?
Alpha decay is a process in which a radioactive nucleus emits an alpha particle.
2. What is an alpha particle?
An alpha particle is composed of two protons and two neutrons, similar to a helium nucleus.
3. How does alpha decay occur?
In alpha decay, the nucleus of an atom undergoes spontaneous decay and emits an alpha particle to become a different element.
4. What are the characteristics of alpha particles?
Alpha particles are positively charged, have a high ionizing power, and their penetration power is limited to a few centimeters of air or a few millimeters of solid material.
5. What is the significance of alpha decay?
Alpha decay is crucial in understanding the stability and decay of radioactive elements, as well as its applications in radiation therapy and nuclear energy.
6. How does half-life relate to alpha decay?
Half-life is the time taken for half of the radioactive atoms in a sample to undergo decay. Alpha decay is one of the processes that contribute to the decay of radioactive substances.
7. Can alpha particles be harmful to living organisms?
Although alpha particles have limited penetration power, they can be harmful if inhaled, ingested, or taken directly into the body. They can cause damage to cells and tissues.
8. Are there any practical applications of alpha particles?
Alpha particles find applications in various fields, including radiation therapy for cancer treatment, smoke detectors, and the generation of nuclear power.
9. How was alpha decay discovered?
Alpha decay was first observed and studied by Ernest Rutherford in the early 20th century during his experiments with radioactive elements.
10. Can alpha decay occur in all elements?
No, alpha decay is typically observed in elements with atomic numbers greater than 83 (bismuth) on the periodic table.
Unraveling alpha decay's mysteries is just the beginning! Dive deeper into radioactive decay's fascinating world with our exploration of its 18 captivating facts. Discover how nuclear reactions shape our universe through 19 mind-blowing revelations about nuclear chemistry. And don't miss out on 8 enigmatic truths about radioactivity that will leave you in awe of this powerful phenomenon. Embark on a journey through the captivating realm of nuclear science and unlock its secrets today!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
