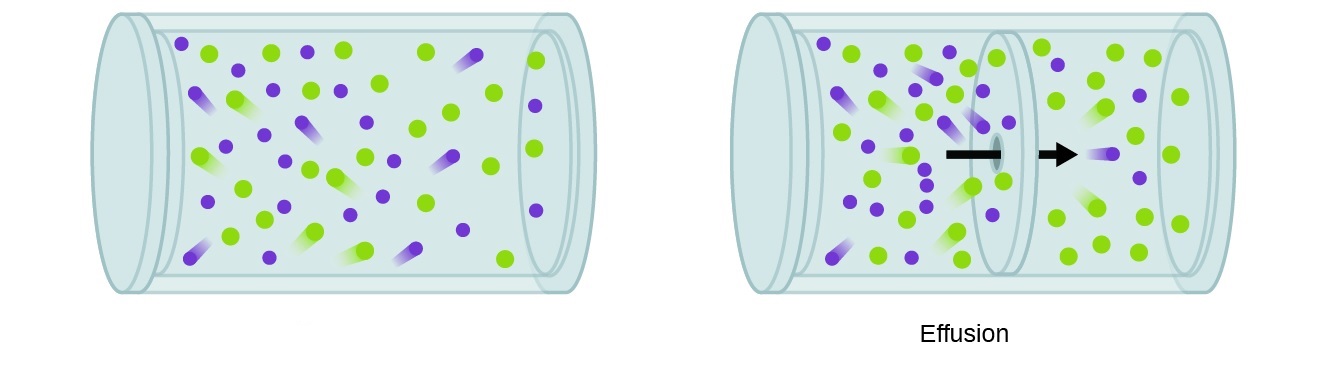
Effusion is a fascinating phenomenon that occurs in the realm of chemistry. It involves the escape of gas molecules through a small opening, often generating intriguing insights and applications. Understanding the principles of effusion can provide scientists with valuable information about molecular behavior, as well as contribute to the development of various technologies and industries.
In this article, we will delve into the captivating world of effusion and explore eight fascinating facts about this process. From its connection to kinetic theory to its role in determining molecular weights, effusion offers a plethora of interesting discoveries. So, let’s embark on our journey and uncover the secrets of effusion.
Key Takeaways:
- Effusion is the escape of gas through a small opening, influencing everything from the hissing sound of opening a soda to the efficiency of gas separation techniques.
- Graham’s Law of Diffusion helps scientists calculate effusion rates, making it possible to design better gas storage and transportation systems for a reliable gas supply.
Effusion is the process of gas escaping through a small opening.
Eager to learn more about this intriguing phenomenon? Let’s dive into the captivating world of effusion.
Effusion is influenced by Graham’s Law of Diffusion.
Graham’s Law states that the rate of effusion is inversely proportional to the square root of the molar mass of the gas. This means that lighter gases effuse faster than heavier ones.
Effusion plays a crucial role in the operation of gas separation techniques.
Effusion is utilized in processes such as gas chromatography and membrane separation to selectively separate different components of a gas mixture based on their rates of effusion.
The concept of effusion is closely related to the concept of diffusion.
While diffusion refers to the movement of gas molecules from an area of high concentration to an area of low concentration, effusion specifically pertains to the escape of gas through a small opening.
Effusion is responsible for the unique hissing sounds produced by pressurized gas escaping from a container.
Next time you open a carbonated beverage, listen carefully to the captivating sound of effusion happening.
Effusion plays a role in everyday life, from the functioning of fuel cells to the inflation of balloons.
Effusion is involved in a range of practical applications, including the efficient conversion of fuel into energy and the inflation of balloons with gas.
The rate of effusion can be mathematically determined using Graham’s Law.
Graham’s Law provides a formula to calculate the rate of effusion based on the molar masses of the gases involved. This allows scientists and engineers to analyze and predict effusion rates in various scenarios.
Understanding effusion helps scientists and engineers design better gas storage and transportation systems.
By studying the principles of effusion, experts can optimize the efficiency and safety of gas storage tanks, pipelines, and other infrastructure, ensuring the reliable supply and distribution of gases.
So there you have it – 8 captivating facts about effusion that shed light on this fascinating process. From its importance in separation techniques to its role in everyday life, effusion remains a topic of interest for researchers and enthusiasts alike.
Conclusion
Effusion is a fascinating phenomenon that plays a crucial role in various fields of chemistry. It involves the escape of molecules through a small opening or hole in a container, resulting in the gradual depletion of the container’s contents. Understanding effusion is essential for applications such as gas separation, vacuum technology, and even in the study of atmospheric pollution.Throughout this article, we explored eight captivating facts about effusion. We learned about Graham’s law and how it relates to the rate of effusion of different gases. We also discovered that effusion can be affected by factors such as temperature and molecular weight. Furthermore, we delved into the concept of effusion in relation to diffusion and explored its significance in the development of the kinetic molecular theory.Effusion is a complex yet intriguing process that continues to pique the interest of scientists and researchers. By studying effusion, we can deepen our understanding of the behavior of gases and their applications in various scientific and industrial settings. So, the next time you come across a container slowly losing its contents, remember the captivating facts about effusion that we’ve uncovered in this article.
FAQs
Q: What is effusion?
A: Effusion is the process by which molecules escape from a container through a small opening or hole.
Q: How does effusion differ from diffusion?
A: Diffusion refers to the spreading of molecules throughout a medium, while effusion specifically refers to the escape of molecules through a small opening.
Q: What is Graham’s law?
A: Graham’s law states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass.
Q: How does temperature affect effusion?
A: With an increase in temperature, the average kinetic energy of the gas molecules increases, leading to higher effusion rates.
Q: Can effusion be used for gas separation?
A: Yes, effusion plays a significant role in gas separation processes, such as in the production of enriched isotopes and the separation of different gases.
Q: Is effusion relevant only in chemistry?
A: Effusion has various applications beyond chemistry, including vacuum technology, environmental studies, and the development of gas sensors.
Q: Can effusion be observed in everyday life?
A: While effusion may not be directly visible in everyday life, it is responsible for the gradual depletion of gas from containers over time.
Q: Why is the study of effusion important?
A: Understanding effusion helps us comprehend the behavior of gases, aids in scientific research, and contributes to the development of technologies that rely on gas properties.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
