Dichlorine monoxide might sound like a mouthful, but it's a fascinating chemical compound with a lot of interesting facts. What is dichlorine monoxide? It's a yellowish gas with the formula Cl2O, known for its strong oxidizing properties. This compound plays a significant role in various chemical reactions and has some surprising uses. From its discovery in the early 19th century to its applications in water treatment and organic synthesis, dichlorine monoxide has a rich history. Whether you're a chemistry enthusiast or just curious, these 40 facts will give you a deeper understanding of this unique substance. Buckle up for a journey through the world of dichlorine monoxide!
Key Takeaways:
- Dichlorine Monoxide: A Reactive Compound Dichlorine monoxide, or chlorine monoxide, is a yellowish gas with a pungent odor. It's highly reactive, soluble in water, and used in bleaching, water treatment, and laboratory research.
- Safety First with Dichlorine Monoxide Handle dichlorine monoxide with care! It's corrosive, can cause respiratory irritation, and contributes to acid rain. Proper protective equipment and storage are crucial to prevent harm and environmental damage.
What is Dichlorine Monoxide?
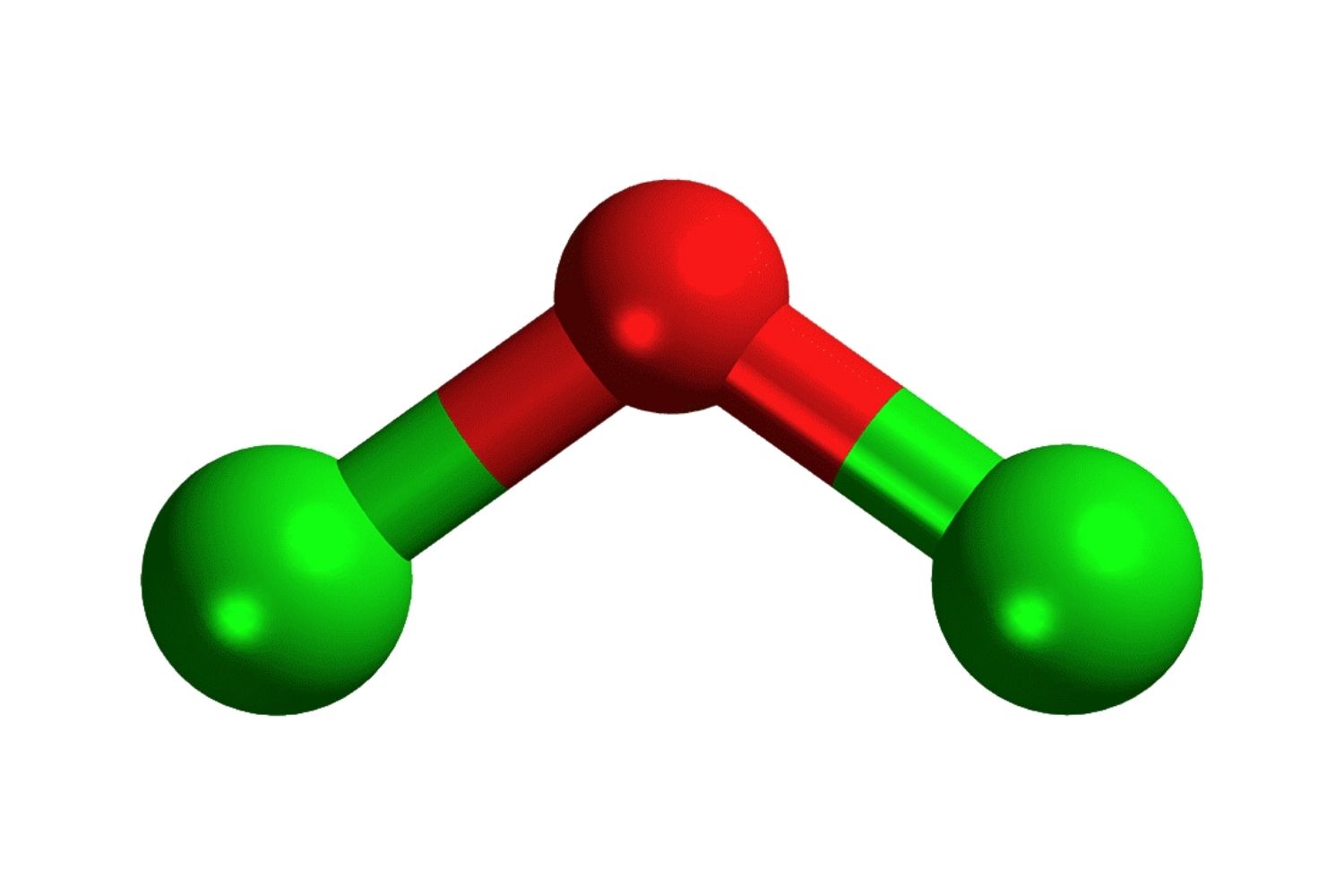
Dichlorine monoxide, also known as chlorine monoxide, is a chemical compound with the formula Cl2O. It is a yellowish gas at room temperature and has some interesting properties and uses. Here are some fascinating facts about this compound.
-
Dichlorine monoxide is a chlorine oxide and is one of the simplest chlorine oxides.
-
It has a pungent odor similar to that of chlorine gas.
-
The compound is highly reactive and can act as an oxidizing agent.
-
Dichlorine monoxide is soluble in water, forming a solution that is acidic due to the formation of hypochlorous acid.
Chemical Properties of Dichlorine Monoxide
Understanding the chemical properties of dichlorine monoxide can help us appreciate its reactivity and potential applications.
-
The molecular structure of dichlorine monoxide is bent, similar to that of water.
-
It has a bond angle of approximately 110 degrees.
-
Dichlorine monoxide is paramagnetic, meaning it has unpaired electrons.
-
It can decompose into chlorine and oxygen when heated.
-
The compound can react with organic compounds, often leading to chlorination.
Production and Synthesis
Dichlorine monoxide is not found naturally and must be synthesized in a laboratory setting.
-
It can be produced by the reaction of chlorine gas with mercury(II) oxide.
-
Another method involves the reaction of chlorine gas with sodium hydroxide at low temperatures.
-
Dichlorine monoxide can also be synthesized by the reaction of chlorine gas with moist sodium carbonate.
-
The compound is often produced in small quantities due to its reactivity and instability.
Uses of Dichlorine Monoxide
Despite its reactivity, dichlorine monoxide has several practical applications.
-
It is used as a bleaching agent in the paper and textile industries.
-
The compound is employed in water treatment to disinfect and kill bacteria.
-
Dichlorine monoxide can be used in the synthesis of other chemicals, such as hypochlorous acid.
-
It is sometimes used in laboratory research to study oxidation reactions.
Safety and Handling
Due to its reactive nature, dichlorine monoxide must be handled with care.
-
Dichlorine monoxide is corrosive and can cause burns upon contact with skin.
-
Inhalation of the gas can lead to respiratory irritation and other health issues.
-
Proper protective equipment, such as gloves and goggles, should be worn when handling the compound.
-
It should be stored in airtight containers to prevent decomposition and reactions with other substances.
Environmental Impact
The environmental impact of dichlorine monoxide is an important consideration.
-
Dichlorine monoxide can contribute to the formation of acid rain when released into the atmosphere.
-
The compound can react with organic matter in water, leading to the formation of harmful byproducts.
-
It is important to control emissions of dichlorine monoxide to minimize environmental damage.
Interesting Facts
Here are some additional intriguing tidbits about dichlorine monoxide.
-
Dichlorine monoxide was first discovered in the early 19th century.
-
The compound has a relatively short half-life in the atmosphere, decomposing quickly.
-
It is one of the few compounds that can oxidize gold.
-
Dichlorine monoxide is sometimes referred to as "chlorine monoxide", though this term can also refer to the radical ClO.
-
The compound has been studied for its potential use in rocket propellants due to its oxidizing properties.
Dichlorine Monoxide in Popular Culture
While not commonly featured, dichlorine monoxide has made some appearances in media and literature.
-
It has been mentioned in science fiction as a potential weapon due to its reactivity.
-
Some educational materials use dichlorine monoxide to illustrate concepts of chemical reactivity and oxidation.
-
The compound has been featured in chemistry demonstrations to show its bleaching properties.
Research and Development
Ongoing research continues to explore the properties and potential uses of dichlorine monoxide.
-
Scientists are investigating its use in advanced oxidation processes for water treatment.
-
Research is being conducted on its potential to decompose pollutants in the environment.
-
New methods of synthesizing dichlorine monoxide are being developed to improve safety and efficiency.
Fun Facts
Let's end with some fun and quirky facts about dichlorine monoxide.
-
Dichlorine monoxide is sometimes called "yellow chlorine" due to its color.
-
The compound can form explosive mixtures with organic materials.
-
It has a boiling point of 2 degrees Celsius, just above the freezing point of water.
-
Dichlorine monoxide can be detected by its distinctive odor, which is similar to that of chlorine.
-
Despite its dangers, dichlorine monoxide remains a fascinating subject of study for chemists around the world.
The Final Word on Dichlorine Monoxide
Dichlorine monoxide, often overlooked, holds a fascinating place in chemistry. This compound, with its unique properties and uses, plays a crucial role in various industrial processes. From its role in water treatment to its use in organic synthesis, dichlorine monoxide proves its worth time and again. Understanding its behavior, safety measures, and applications can help us appreciate its significance. Whether you're a student, a professional, or just curious, knowing these facts can broaden your knowledge and spark interest in the world of chemistry. So next time you hear about dichlorine monoxide, you'll know it's more than just a chemical formula. It's a vital part of many processes that impact our daily lives. Keep exploring, stay curious, and never stop learning about the wonders of science.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
