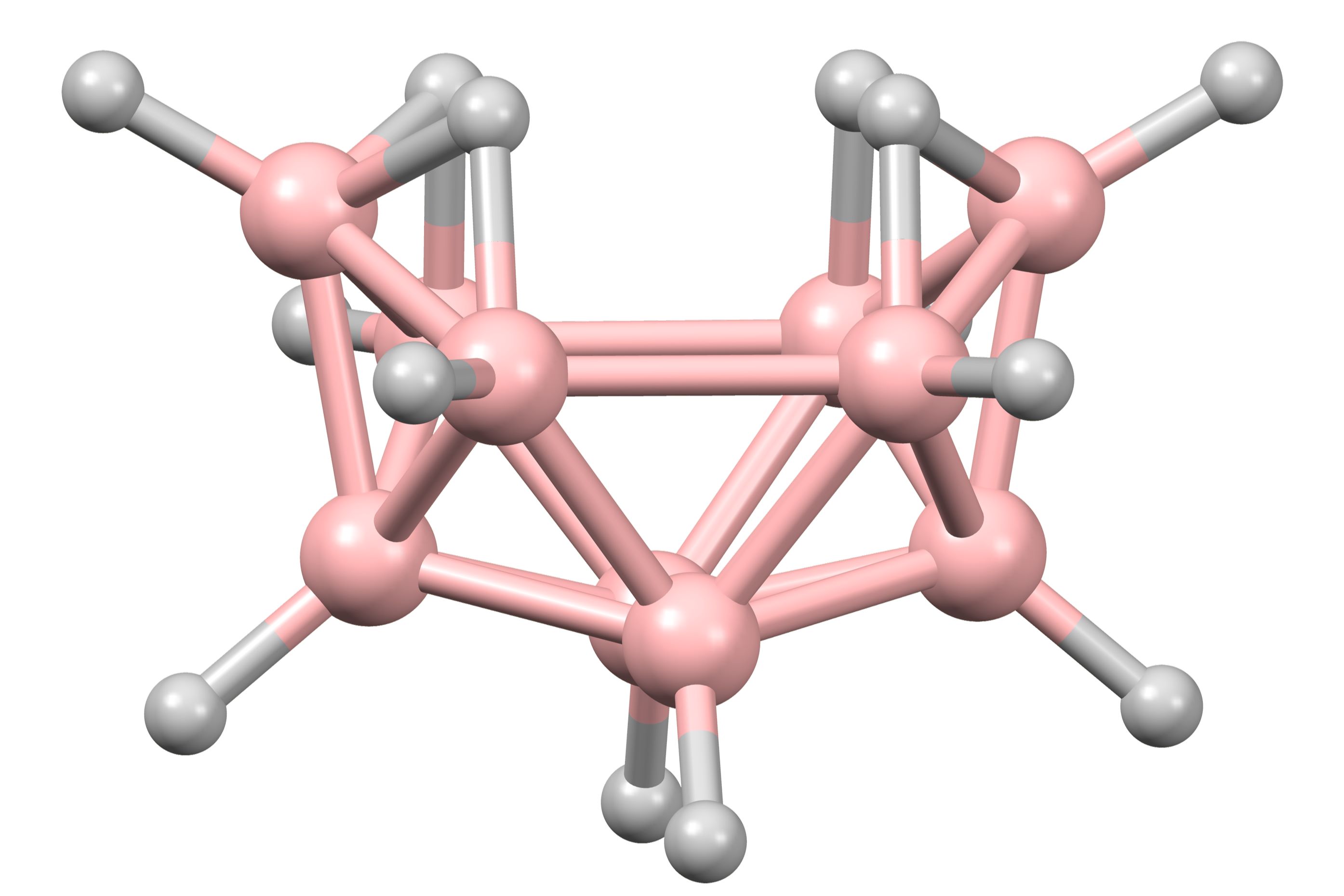
Decaborane is a fascinating chemical compound with a unique structure and intriguing properties. But what exactly is decaborane? Decaborane is a boron-hydrogen compound, specifically B10H14, known for its use in rocket fuel, chemical synthesis, and even medical applications. This compound stands out due to its stability and high energy content. Its molecular structure resembles a cage, making it a subject of interest for chemists and researchers. Decaborane also plays a role in the semiconductor industry, where it aids in doping processes. Whether you're a science enthusiast or just curious, these 40 facts about decaborane will shed light on its many uses and characteristics.
Key Takeaways:
- Decaborane is a unique chemical compound with diverse applications, from rocket fuel to potential cancer treatment. Its reactivity and distinctive properties make it a fascinating subject of study in various fields.
- Despite its potential, decaborane requires careful handling due to its toxicity and fire hazard. Ongoing research aims to explore new uses while improving safety measures for this intriguing compound.
What is Decaborane?
Decaborane is a fascinating chemical compound with the formula B₁₀H₁₄. It has unique properties and applications that make it a subject of interest in various fields, from chemistry to aerospace. Let's dive into some intriguing facts about this compound.
-
Decaborane's molecular formula is B₁₀H₁₄. This means it consists of ten boron atoms and fourteen hydrogen atoms.
-
It belongs to the boranes family. Boranes are compounds of boron and hydrogen, known for their diverse structures and bonding.
-
Decaborane appears as a white crystalline solid. Its appearance can be deceiving, as it looks quite ordinary despite its unique properties.
-
It has a melting point of 99.6°C. This relatively low melting point makes it easy to handle in various applications.
-
The boiling point of decaborane is 213°C. This is significantly higher than its melting point, indicating a wide liquid range.
Chemical Properties of Decaborane
Understanding the chemical properties of decaborane helps in grasping its reactivity and potential uses.
-
Decaborane is highly reactive with oxygen. It can ignite spontaneously in air, making it a fire hazard.
-
It reacts with water to form boric acid and hydrogen gas. This reaction is exothermic, releasing a significant amount of heat.
-
Decaborane can act as a Lewis acid. It can accept electron pairs, making it useful in various chemical reactions.
-
It forms complexes with amines. These complexes are often used in organic synthesis.
-
Decaborane can be reduced to form smaller boranes. This reduction process is useful in studying the chemistry of boron-hydrogen compounds.
Uses of Decaborane
Decaborane's unique properties make it valuable in several industries and scientific research.
-
It is used as a rocket fuel additive. Its high energy content makes it suitable for enhancing the performance of rocket propellants.
-
Decaborane serves as a precursor in the synthesis of other boron compounds. These compounds have applications in materials science and medicine.
-
It is used in the semiconductor industry. Decaborane is employed in doping processes to modify the electrical properties of semiconductors.
-
Decaborane has potential applications in cancer treatment. Research is ongoing to explore its use in boron neutron capture therapy (BNCT).
-
It is used in the production of boron carbide. Boron carbide is a hard material used in armor and cutting tools.
Safety and Handling of Decaborane
Due to its reactivity, decaborane must be handled with care to ensure safety.
-
Decaborane is toxic if inhaled or ingested. Proper protective equipment is necessary when working with it.
-
It should be stored in a cool, dry place. This minimizes the risk of accidental ignition or decomposition.
-
Decaborane can cause skin and eye irritation. Direct contact should be avoided to prevent injury.
-
It should be handled in a well-ventilated area. This reduces the risk of inhaling harmful fumes.
-
Emergency procedures should be in place when working with decaborane. Quick response can mitigate the effects of accidental exposure.
Historical Context of Decaborane
The history of decaborane provides insight into its development and significance.
-
Decaborane was first synthesized in the 1950s. Its discovery opened new avenues in boron chemistry.
-
It played a role in early rocket research. Scientists explored its potential as a high-energy fuel component.
-
Decaborane has been studied extensively for its unique bonding. The compound's structure challenged traditional views of chemical bonding.
-
It contributed to the development of boron chemistry. Research on decaborane led to the discovery of other boron-hydrogen compounds.
-
Decaborane's synthesis methods have evolved over time. Modern techniques allow for more efficient and safer production.
Interesting Facts about Decaborane
Here are some lesser-known but fascinating tidbits about decaborane.
-
Decaborane has a distinctive odor. It smells somewhat like camphor, making it easily recognizable.
-
It can form clathrate compounds. These are structures where decaborane molecules are trapped within a lattice of another substance.
-
Decaborane is used in neutron detection. Its boron content makes it effective in capturing neutrons.
-
It has been studied for potential use in hydrogen storage. Decaborane's high hydrogen content makes it a candidate for this application.
-
Decaborane can be used in organic synthesis. It serves as a reagent in various chemical reactions.
Future Prospects of Decaborane
The future holds exciting possibilities for decaborane, with ongoing research exploring new applications.
-
Research is exploring its use in nanotechnology. Decaborane's unique properties could lead to advancements in this field.
-
It may play a role in future energy solutions. Its potential in hydrogen storage and high-energy fuels is being investigated.
-
Decaborane could contribute to advancements in medicine. Its use in BNCT and other therapies is a promising area of study.
-
It may find new applications in materials science. Decaborane's reactivity and bonding properties make it a valuable tool for creating new materials.
-
Ongoing research aims to improve its safety and handling. Developing safer methods for working with decaborane will expand its usability.
Fun Facts about Decaborane
Let's end with some fun and quirky facts about this intriguing compound.
-
Decaborane can form beautiful crystals. These crystals are often used in educational demonstrations.
-
It has been featured in science fiction. Decaborane's unique properties have inspired imaginative uses in literature and film.
-
Decaborane's structure is a topic of study in chemistry classes. Its bonding and geometry provide valuable lessons for students.
-
It has a role in forensic science. Decaborane can be used in certain types of chemical analysis.
-
Decaborane's name comes from its composition. "Deca" means ten, referring to the ten boron atoms in its structure.
Final Thoughts on Decaborane
Decaborane, a fascinating compound, holds a unique spot in chemistry. Known for its boron and hydrogen composition, it’s used in rocket fuels, semiconductors, and neutron capture therapy. Despite its benefits, handling decaborane requires caution due to its toxicity and flammability. Its structure, resembling a boron cage, showcases the complexity and beauty of chemical compounds. Understanding decaborane not only broadens our knowledge of chemistry but also highlights the importance of safety in scientific exploration. Whether you’re a student, a professional, or just curious, learning about decaborane offers a glimpse into the intricate world of chemical compounds. Stay curious, stay safe, and keep exploring the wonders of science.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
