Chlorosulfonyl isocyanate might sound like a mouthful, but this chemical compound packs a punch in the world of chemistry. Known for its reactive nature, it plays a crucial role in various industrial applications. But what exactly is chlorosulfonyl isocyanate? It is a highly reactive compound used in organic synthesis, particularly in the production of pharmaceuticals and agrochemicals. This versatile chemical can be both a friend and a foe, depending on how it's handled. From its discovery to its practical uses, chlorosulfonyl isocyanate has a fascinating story. Ready to dive into some intriguing facts about this compound? Let's get started!
Key Takeaways:
- Chlorosulfonyl isocyanate (CSI) is a versatile chemical compound used in various industries for making medicines, polymers, and agricultural chemicals. It has a pungent odor and requires strict safety measures when handling.
- CSI is known for its reactivity and participation in various chemical reactions. It can decompose into non-toxic products when exposed to moisture, but it is toxic to aquatic life and requires careful disposal.
What is Chlorosulfonyl Isocyanate?
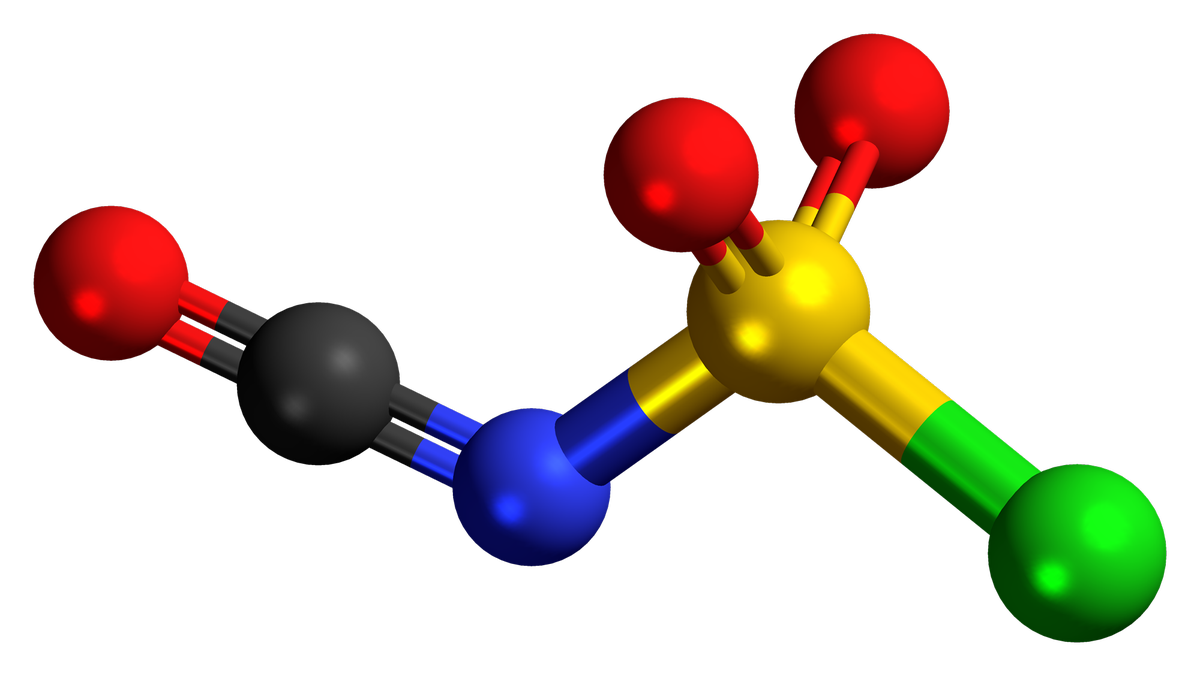
Chlorosulfonyl isocyanate (CSI) is a versatile chemical compound used in various industrial applications. Known for its reactivity, CSI plays a crucial role in organic synthesis and material science.
- Chemical Formula: CSI has the chemical formula ClSO2NCO.
- Appearance: This compound appears as a colorless to pale yellow liquid.
- Odor: CSI has a pungent odor, making it easily identifiable.
Uses of Chlorosulfonyl Isocyanate
CSI's unique properties make it valuable in multiple fields. Here are some of its primary uses:
- Organic Synthesis: CSI is widely used in organic synthesis to introduce sulfonyl and isocyanate groups into molecules.
- Pharmaceuticals: It plays a role in the production of pharmaceuticals, particularly in creating intermediates.
- Polymers: CSI is used in the manufacture of polymers, enhancing their properties.
- Agricultural Chemicals: It is also utilized in the synthesis of agrochemicals.
Safety and Handling
Handling CSI requires strict safety measures due to its reactivity and potential hazards.
- Protective Gear: Always wear protective clothing, gloves, and goggles when handling CSI.
- Ventilation: Ensure adequate ventilation in the workspace to avoid inhaling fumes.
- Storage: Store CSI in a cool, dry place away from incompatible substances.
Chemical Reactions
CSI is known for its reactivity, participating in various chemical reactions.
- Hydrolysis: CSI reacts with water to form sulfonyl chloride and carbon dioxide.
- Amination: It reacts with amines to produce ureas and sulfonamides.
- Alcohol Reaction: CSI reacts with alcohols to form carbamates.
Environmental Impact
Understanding the environmental impact of CSI is crucial for its safe use.
- Decomposition: CSI decomposes into non-toxic products when exposed to moisture.
- Toxicity: It is toxic to aquatic life, necessitating careful disposal.
- Biodegradability: CSI is not readily biodegradable, requiring proper waste management.
Historical Background
CSI has an interesting history in the field of chemistry.
- Discovery: CSI was first synthesized in the early 20th century.
- Industrial Use: Its industrial applications expanded significantly in the mid-20th century.
- Research: Ongoing research continues to explore new uses for CSI.
Physical Properties
CSI's physical properties are key to its functionality.
- Boiling Point: CSI has a boiling point of 152°C.
- Density: The density of CSI is 1.63 g/cm³.
- Solubility: It is soluble in organic solvents like benzene and toluene.
Health Hazards
Exposure to CSI can pose health risks, making awareness essential.
- Inhalation: Inhaling CSI can cause respiratory irritation.
- Skin Contact: Direct contact with skin can lead to burns and irritation.
- Eye Contact: CSI can cause severe eye damage upon contact.
Industrial Applications
CSI's versatility extends to various industrial applications.
- Dye Manufacturing: It is used in the production of dyes.
- Textile Industry: CSI helps in the treatment of textiles.
- Plastic Industry: It is employed in the modification of plastics.
Regulatory Aspects
Regulations govern the use and handling of CSI to ensure safety.
- OSHA Guidelines: The Occupational Safety and Health Administration provides guidelines for handling CSI.
- EPA Regulations: The Environmental Protection Agency regulates its disposal.
- International Standards: CSI use is subject to international chemical safety standards.
Future Prospects
Research and development continue to expand CSI's potential applications.
- Nanotechnology: CSI is being explored for use in nanotechnology.
- Green Chemistry: Efforts are underway to make CSI processes more environmentally friendly.
- Medical Research: CSI's role in medical research is growing, particularly in drug development.
Fun Facts
Here are some interesting tidbits about CSI that you might not know.
- Nicknames: CSI is sometimes referred to as "CSI gas" due to its gaseous state at room temperature.
- Pop Culture: Despite its industrial use, CSI has made appearances in science fiction literature.
- Color Change: CSI can change color when exposed to certain chemicals.
Miscellaneous Information
Additional facts that don't fit into other categories but are still fascinating.
- Patents: Numerous patents involve CSI, highlighting its importance in innovation.
- Educational Use: CSI is often used in educational demonstrations to teach chemical reactions.
- Global Production: CSI is produced globally, with major manufacturers in Europe, Asia, and North America.
Final Thoughts on Chlorosulfonyl Isocyanate
Chlorosulfonyl isocyanate, a fascinating compound, plays a crucial role in various chemical reactions. Its unique properties make it invaluable in pharmaceuticals, agrochemicals, and polymer industries. Handling this compound requires caution due to its reactivity and potential hazards. Yet, its benefits in creating complex molecules can't be overstated.
Understanding chlorosulfonyl isocyanate's applications and safety measures ensures its effective and safe use. This knowledge empowers chemists and industry professionals to harness its full potential while minimizing risks. So, whether you're a student, researcher, or industry expert, grasping these facts about chlorosulfonyl isocyanate can enhance your work and safety practices.
Stay curious and informed about the chemicals shaping our world. Knowledge is power, especially when dealing with such potent compounds. Keep exploring, learning, and applying this information to make informed decisions in your chemical endeavors.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
