What is n-Butane? n-Butane, a colorless gas, is part of the alkane family. It’s commonly found in natural gas and petroleum. This hydrocarbon has the chemical formula C4H10. Why is n-Butane important? It’s used in various applications, from fuel for lighters to a refrigerant in cooling systems. How does n-Butane impact daily life? You might not realize it, but n-Butane plays a role in many everyday products. Is n-Butane safe? When handled properly, it’s generally safe, but it can be hazardous if misused. Why should you care about n-Butane? Understanding its uses and safety can help you make informed choices. Ready to learn more? Let’s dive into 30 fascinating facts about n-Butane!
What is n-Butane?
n-Butane is a colorless, flammable gas that belongs to the alkane family. It is commonly used as a fuel, refrigerant, and in the production of synthetic rubber. Let's dive into some fascinating facts about this versatile compound.
-
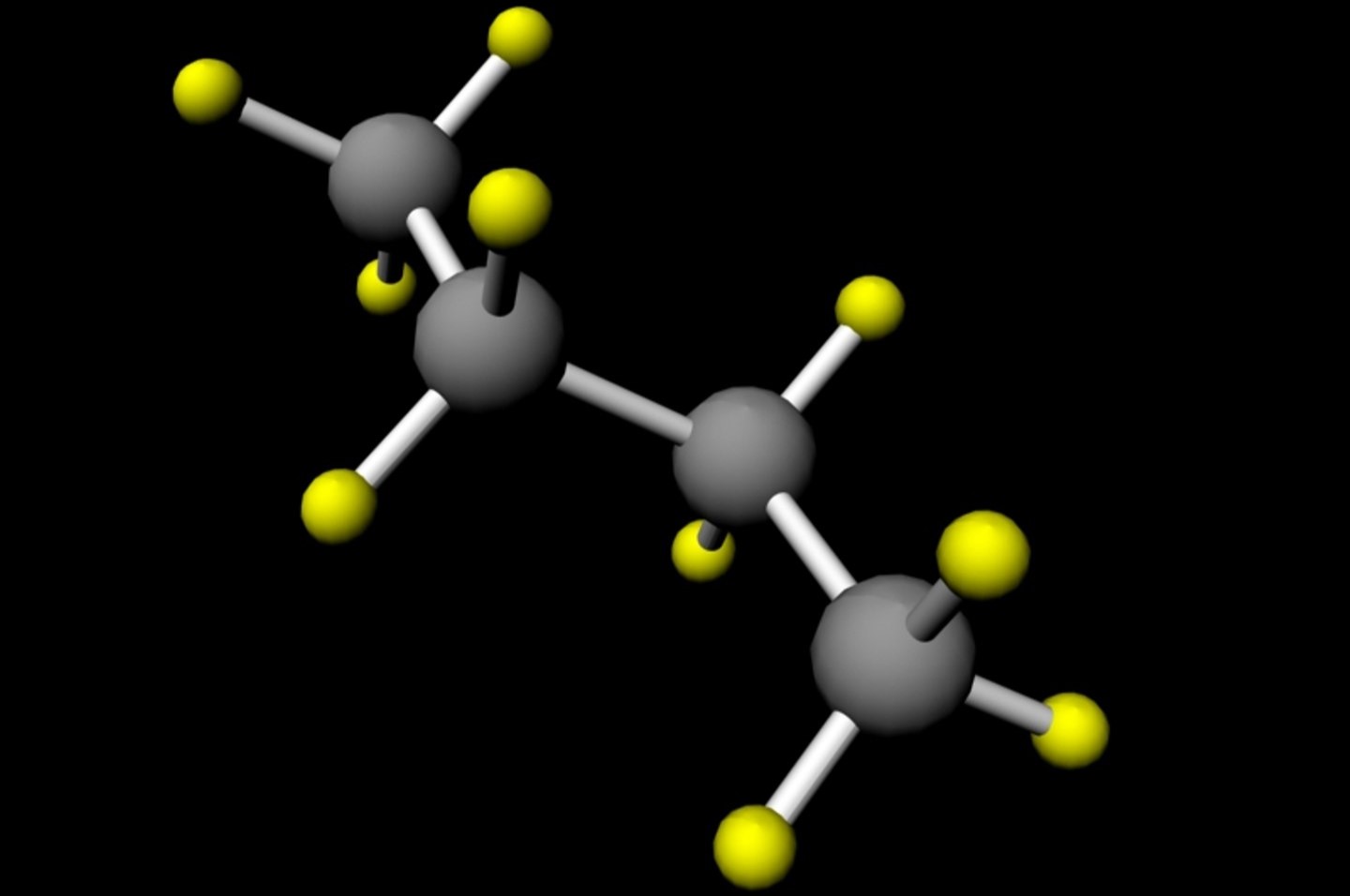
Chemical Formula: n-Butane has the chemical formula C4H10, indicating it consists of four carbon atoms and ten hydrogen atoms.
-
Structure: The structure of n-Butane is linear, meaning all carbon atoms are connected in a straight chain.
-
Boiling Point: n-Butane boils at -0.5°C (31.1°F), making it a gas at room temperature.
-
Density: It has a density of approximately 2.48 kg/m³ at 15°C, which is heavier than air.
-
Natural Gas Component: n-Butane is a significant component of natural gas and is often extracted during natural gas processing.
Uses of n-Butane
n-Butane has a wide range of applications in various industries. Here are some of its primary uses:
-
Fuel: n-Butane is commonly used as a fuel in lighters and portable stoves.
-
Refrigerant: It serves as a refrigerant in some air conditioning and refrigeration systems.
-
Aerosol Propellant: Many aerosol sprays use n-Butane as a propellant due to its low toxicity and high vapor pressure.
-
Synthetic Rubber Production: n-Butane is a key ingredient in the production of synthetic rubber, which is used in tires and other rubber products.
-
Petrochemical Feedstock: It is used as a feedstock in the petrochemical industry to produce various chemicals and plastics.
Safety and Handling
Handling n-Butane requires caution due to its flammability and potential health effects. Here are some important safety facts:
-
Flammability: n-Butane is highly flammable and can form explosive mixtures with air.
-
Storage: It should be stored in well-ventilated areas away from heat sources and open flames.
-
Health Effects: Inhalation of n-Butane can cause dizziness, headaches, and in severe cases, asphyxiation.
-
First Aid: In case of inhalation, move the person to fresh air immediately and seek medical attention if symptoms persist.
-
Firefighting: Use dry chemical powder, carbon dioxide, or foam to extinguish n-Butane fires.
Environmental Impact
Understanding the environmental impact of n-Butane is crucial for its responsible use. Here are some key points:
-
Greenhouse Gas: n-Butane is a greenhouse gas, contributing to global warming when released into the atmosphere.
-
Ozone Depletion: Unlike some other refrigerants, n-Butane does not deplete the ozone layer.
-
Biodegradability: It is not readily biodegradable, meaning it can persist in the environment for a long time.
-
Air Quality: Emissions of n-Butane can contribute to air pollution and smog formation.
-
Regulations: Many countries have regulations in place to limit n-Butane emissions and ensure safe handling.
Interesting Facts
Here are some lesser-known but intriguing facts about n-Butane:
-
Discovery: n-Butane was first isolated by the chemist Edward Frankland in 1849.
-
Isomers: n-Butane has an isomer called isobutane, which has a branched structure.
-
Odor: Pure n-Butane is odorless, but a foul-smelling odorant is often added for leak detection.
-
Energy Density: It has a high energy density, making it an efficient fuel source.
-
Common Mixtures: n-Butane is often mixed with propane to create liquefied petroleum gas (LPG).
Industrial Production
The production of n-Butane involves several industrial processes. Here are some key aspects:
-
Fractional Distillation: n-Butane is separated from natural gas and crude oil through fractional distillation.
-
Cracking: It can also be produced by cracking larger hydrocarbons in petroleum refineries.
-
Purification: The extracted n-Butane undergoes purification to remove impurities and ensure high quality.
-
Storage: Large quantities of n-Butane are stored in pressurized tanks for industrial use.
-
Transportation: It is transported in liquefied form via pipelines, tankers, and railcars.
The Final Word on n-Butane
n-Butane, a versatile hydrocarbon, plays a crucial role in various industries. From fueling lighters to serving as a refrigerant, its applications are vast. This colorless gas, with a faint petroleum-like odor, is not just limited to everyday uses. It's also a key player in the petrochemical industry, where it's used to produce synthetic rubber and other chemicals.
Safety is paramount when handling n-Butane. It's highly flammable and can pose health risks if inhaled in large quantities. Proper storage and handling are essential to prevent accidents.
Understanding n-Butane's properties and uses can help you appreciate its importance in our daily lives. Whether you're a student, a professional, or just curious, knowing these facts can provide valuable insights into this common yet fascinating compound. Stay informed and stay safe!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


