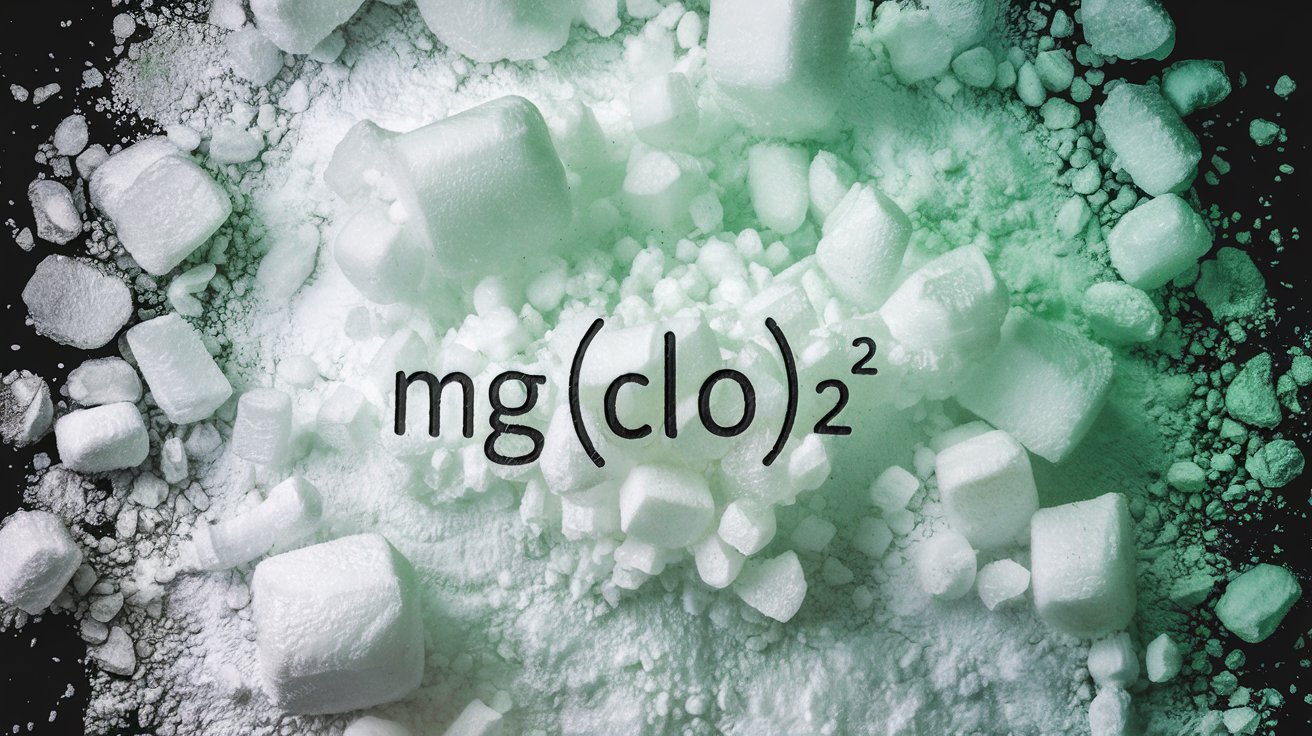
Magnesium hypochlorite might sound like a mouthful, but it's a fascinating compound with many uses. Ever wondered how swimming pools stay clean or how certain disinfectants work? This chemical plays a big role in those processes. Magnesium hypochlorite is a powerful oxidizing agent, meaning it helps break down unwanted substances. It's often used in water treatment, sanitation, and even some industrial applications. But there's more to it than just its cleaning power. From its chemical structure to its practical applications, this compound has a lot to offer. Ready to dive into some intriguing facts about magnesium hypochlorite? Let's get started!
Key Takeaways:
- Magnesium hypochlorite, a white powder, is used for disinfection and bleaching. It's soluble in water and acts as a strong oxidizing agent, making it effective in killing bacteria and viruses.
- This chemical compound is used in water treatment, swimming pools, textile industry, and agriculture. It's important to handle it with caution, as it can release chlorine gas when decomposed.
What is Magnesium Hypochlorite?
Magnesium hypochlorite is a chemical compound often used for disinfection and bleaching. It’s less common than other hypochlorites but has unique properties worth knowing.
-
Chemical Formula: The chemical formula for magnesium hypochlorite is Mg(OCl)₂. This indicates it contains magnesium, oxygen, and chlorine.
-
Appearance: Typically, magnesium hypochlorite appears as a white powder. This makes it easy to identify and handle.
-
Solubility: It is soluble in water. This property is crucial for its use in disinfection and bleaching.
-
Oxidizing Agent: Magnesium hypochlorite acts as a strong oxidizing agent. This means it can easily donate oxygen to other substances, making it effective in killing bacteria and viruses.
Uses of Magnesium Hypochlorite
Magnesium hypochlorite has various applications, particularly in sanitation and industrial processes.
-
Water Treatment: It is used in water treatment plants to disinfect drinking water. This helps ensure the water is safe for consumption.
-
Swimming Pools: Often used to keep swimming pools clean. It helps eliminate harmful microorganisms, making the water safe for swimmers.
-
Bleaching Agent: In the textile industry, magnesium hypochlorite is used as a bleaching agent. It helps in whitening fabrics.
-
Agriculture: Farmers use it to disinfect irrigation water. This helps prevent the spread of plant diseases.
Safety and Handling
Handling chemicals like magnesium hypochlorite requires caution. Here are some safety tips and facts.
-
Protective Gear: Always wear protective gear, such as gloves and goggles, when handling magnesium hypochlorite. This prevents skin and eye irritation.
-
Storage: Store it in a cool, dry place. Exposure to moisture can cause it to decompose, releasing chlorine gas.
-
Inhalation Hazard: Inhaling magnesium hypochlorite dust can irritate the respiratory system. Always handle it in a well-ventilated area.
-
First Aid: If it comes into contact with skin, wash the area thoroughly with water. Seek medical attention if irritation persists.
Environmental Impact
Understanding the environmental impact of magnesium hypochlorite is essential for responsible use.
-
Decomposition: When it decomposes, it releases chlorine gas. This can be harmful to the environment if not managed properly.
-
Aquatic Life: High concentrations can be toxic to aquatic life. It’s crucial to use it in controlled amounts to prevent environmental damage.
-
Biodegradability: Magnesium hypochlorite is not biodegradable. This means it can persist in the environment if not disposed of correctly.
Chemical Reactions
Magnesium hypochlorite participates in various chemical reactions, making it versatile in different applications.
-
Reaction with Acids: When mixed with acids, it releases chlorine gas. This reaction is useful in some industrial processes but requires careful handling.
-
Decomposition by Heat: Heating magnesium hypochlorite causes it to decompose, releasing oxygen and chlorine gas. This property is used in some chemical manufacturing processes.
-
Reaction with Organic Compounds: It reacts with organic compounds, making it effective in disinfection. This reaction helps break down harmful microorganisms.
Historical Context
The history of magnesium hypochlorite provides insight into its development and use over time.
-
Discovery: Magnesium hypochlorite was discovered in the early 20th century. Its unique properties quickly made it valuable in various industries.
-
Industrial Use: During World War II, it was used extensively for disinfecting water supplies. This helped prevent the spread of diseases among soldiers.
-
Modern Applications: Today, it continues to be used in water treatment, agriculture, and industry. Its effectiveness and versatility make it a valuable chemical.
Comparisons with Other Hypochlorites
Comparing magnesium hypochlorite with other hypochlorites helps understand its unique properties.
-
Calcium Hypochlorite: Unlike calcium hypochlorite, magnesium hypochlorite is less commonly used but offers similar disinfecting properties.
-
Sodium Hypochlorite: Sodium hypochlorite, commonly known as bleach, is more widely used. However, magnesium hypochlorite is preferred in some specific applications due to its unique properties.
-
Potassium Hypochlorite: Potassium hypochlorite is another alternative. Magnesium hypochlorite is often chosen for its effectiveness in certain industrial processes.
Fun Facts
Here are some interesting tidbits about magnesium hypochlorite that you might find surprising.
-
Chlorine Source: It serves as a source of chlorine in some chemical reactions. This makes it valuable in various manufacturing processes.
-
Stability: Magnesium hypochlorite is more stable than some other hypochlorites. This stability makes it easier to store and handle.
-
Cost: It is generally more expensive than other hypochlorites. This higher cost is due to its unique properties and specific applications.
-
Color Change: When it decomposes, it can cause a color change in the solution. This property is sometimes used as an indicator in chemical reactions.
-
Odor: It has a strong chlorine odor. This odor can be a useful indicator of its presence and concentration.
-
Household Use: While not common, it can be used in household cleaning products. Its disinfecting properties make it effective in killing germs and bacteria.
The Final Scoop on Magnesium Hypochlorite
Magnesium hypochlorite is a real game-changer in water treatment and sanitation. Its strong disinfecting properties make it a go-to for keeping pools, drinking water, and even wastewater safe. This compound, with its powerful oxidizing abilities, helps eliminate harmful bacteria and viruses, ensuring clean and safe water for everyone.
Not only does it work wonders in water treatment, but it also plays a role in various industrial processes. From bleaching textiles to aiding in chemical reactions, magnesium hypochlorite proves its versatility time and again.
Understanding its benefits and applications can help you appreciate the science behind clean water and effective sanitation. So, next time you take a dip in a pool or drink a glass of water, remember the unsung hero working behind the scenes—magnesium hypochlorite.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
