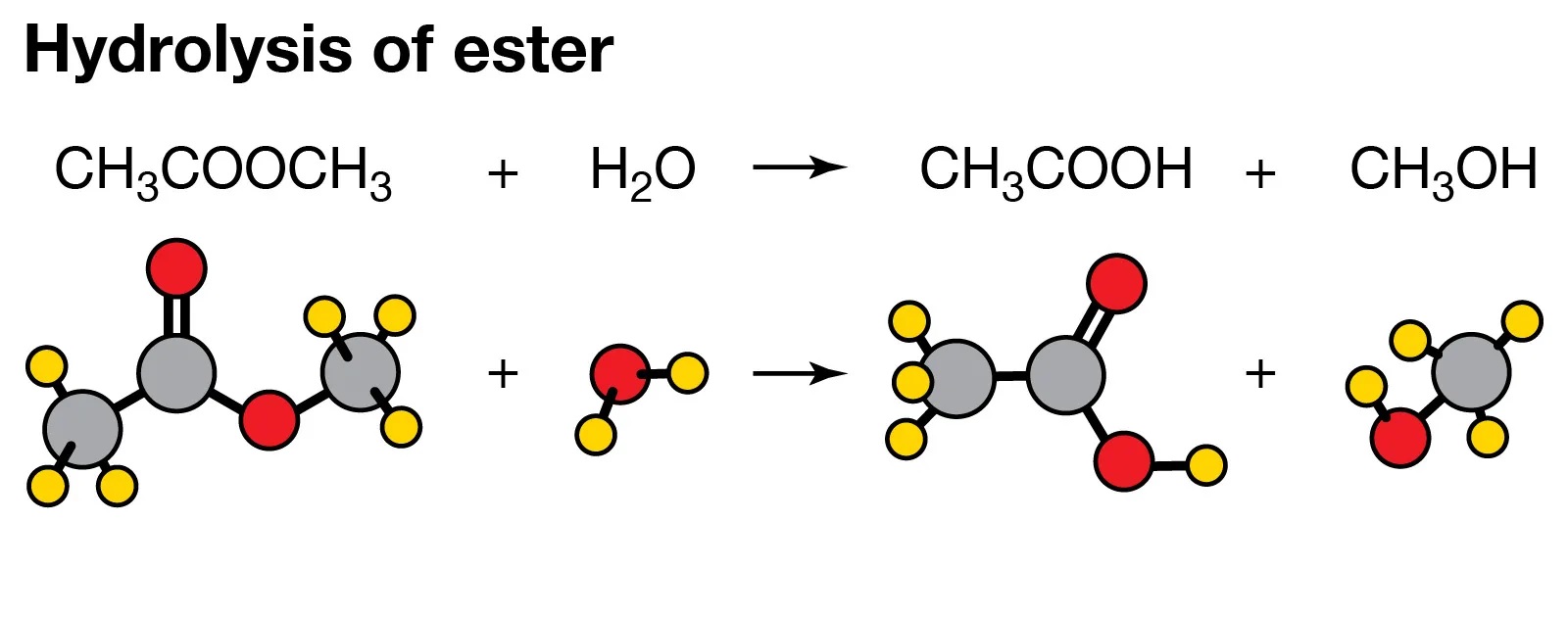
Hydrolysis is a fundamental chemical process that occurs in many different contexts, from the human body to industrial applications. It involves the breaking down of molecules through the addition of water molecules. This crucial reaction plays a crucial role in various fields, including biochemistry, medicine, and environmental science. Understanding the mechanisms and effects of hydrolysis is essential for researchers, scientists, and professionals in these fields.
In this article, we will delve into the fascinating world of hydrolysis and uncover 15 unbelievable facts about this chemical process. From its role in digestion to its impact on drug stability, the discoveries surrounding hydrolysis are bound to astonish you. So fasten your seatbelts and get ready to be amazed by the incredible world of hydrolysis!
Key Takeaways:
- Hydrolysis is a super important chemical process that helps break down food, make soap, and even create paper. It’s like a superhero for the environment and our bodies!
- Enzymes are like the sidekicks that help hydrolysis work faster and better. They make everything from making medicine to cleaning stains possible.
Hydrolysis is a vital process in digestion.
The human body relies on hydrolysis to break down complex carbohydrates, proteins, and fats into simpler molecules that can be easily absorbed and utilized by the body.
It is involved in the synthesis of DNA and RNA.
During DNA and RNA synthesis, hydrolysis reactions are used to link nucleotides together, forming the backbone of these essential genetic materials.
Hydrolysis is responsible for the decomposition of polymers.
Polymers, such as plastics, can undergo hydrolysis when exposed to water and heat, resulting in their degradation and breakdown into smaller, more manageable fragments.
It is used in the production of biodiesel.
Triglycerides, present in vegetable oils and animal fats, can be converted into biodiesel through hydrolysis, providing a sustainable alternative to traditional fossil fuels.
It plays a role in the formation of soap.
The saponification process involves the hydrolysis of fats or oils with an alkali, resulting in the production of soap and glycerol.
Hydrolysis is at the core of the water cycle.
As part of the natural water cycle, hydrolysis reactions occur when water molecules break down complex minerals and rocks, releasing essential nutrients for plants and ecosystems.
It is involved in the degradation of cellular waste.
In cells, hydrolysis reactions break down unnecessary or damaged molecules, allowing for their proper disposal and recycling.
Hydrolysis can be catalyzed by enzymes.
Enzymes facilitate hydrolysis by lowering the activation energy required for the reaction to occur, enhancing the efficiency of these crucial processes.
It is used in the production of paper.
The hydrolysis of cellulose fibers in wood helps break them down into individual cellulose molecules, which are then used to create paper products.
Hydrolysis is utilized in the brewing and fermentation processes.
The breakdown of complex sugars into simpler compounds through hydrolysis is fundamental in the production of alcoholic beverages like beer and wine.
It plays a role in the digestion of lactose.
Lactose hydrolysis is necessary for individuals who are lactose intolerant, as it allows them to break down lactose into its more easily digestible components.
Hydrolysis can aid in the removal of stains.
In laundry detergents, hydrolysis assists in breaking down tough stains by breaking their chemical bonds, making them easier to remove during washing.
It is involved in the weathering of rocks.
Hydrolysis reactions contribute to the gradual breakdown and erosion of rocks over time, shaping the Earth’s surface and creating new landscapes.
Hydrolysis is used in the production of pharmaceuticals.
Pharmaceutical chemists employ hydrolysis reactions to synthesize and modify compounds, crucial for the development of various medications and treatments.
It is a vital step in the metabolism of carbohydrates.
Hydrolysis breaks down complex carbohydrates, such as starch and glycogen, into their monosaccharide components, providing a source of energy for the body.
These 15 incredible facts highlight the profound impact of hydrolysis in various aspects of our lives. From digestion and synthesis to industry and environmental processes, hydrolysis continues to shape the world around us.
Conclusion
Hydrolysis is a fascinating chemical process that plays a crucial role in a wide range of biological and industrial applications. Throughout this article, we have explored 15 unbelievable facts about hydrolysis that showcase its significance and complexity.
From its role in breaking down complex molecules like carbohydrates, proteins, and lipids into smaller, more manageable compounds, to its importance in the production of renewable energy sources such as biodiesel, hydrolysis is a fundamental process that drives numerous biological and chemical reactions.
Understanding the mechanisms and applications of hydrolysis allows us to develop innovative solutions in fields such as medicine, agriculture, and environmental science. By harnessing the power of hydrolysis, we can unlock new possibilities for sustainable and efficient processes.
Whether you are a chemistry enthusiast, a student, or simply curious about the wonders of the natural world, delving into the realm of hydrolysis will inspire awe and appreciation for the intricate workings of chemical reactions.
FAQs
1. What is hydrolysis?
Hydrolysis is a chemical reaction where water molecules break down larger molecules into smaller compounds by breaking the chemical bonds holding them together.
2. What are some examples of hydrolysis reactions?
Examples of hydrolysis reactions include the breaking down of starch into glucose, the breakdown of proteins into amino acids, and the hydrolysis of fats into glycerol and fatty acids.
3. How does hydrolysis occur in the human body?
Hydrolysis is an essential process in digestion, as it helps break down complex molecules in food into simpler forms that the body can absorb and utilize.
4. What are the industrial applications of hydrolysis?
Hydrolysis is widely used in industries such as biofuel production, pharmaceutical manufacturing, and the production of detergents and cleaning agents.
5. How does hydrolysis contribute to the water cycle?
Hydrolysis plays a crucial role in the water cycle by breaking down water molecules into hydrogen and oxygen, allowing them to cycle through the environment.
6. Can hydrolysis be reversed?
In some cases, hydrolysis reactions can be reversed through a process called condensation or dehydration synthesis, where smaller molecules combine to form larger ones.
7. Are there any risks associated with hydrolysis reactions?
While hydrolysis is a natural process, certain reactions can be dangerous if not conducted properly. Some substances may release toxic or flammable gases during hydrolysis.
8. How is hydrolysis related to pH?
The rate of hydrolysis reactions can be influenced by the acidity (pH) of the solution. A higher pH typically increases the rate of hydrolysis.
9. Are there any enzymes involved in hydrolysis?
Yes, enzymes called hydrolases facilitate hydrolysis reactions by speeding up the breakdown of specific molecules.
10. Can hydrolysis occur in non-aqueous solutions?
While hydrolysis is commonly associated with water, it can occur in non-aqueous solvents, such as sulfuric acid or supercritical carbon dioxide.
Hydrolysis is truly a remarkable process, with its far-reaching effects in various aspects of life. From its role in digestion to its industrial applications, understanding hydrolysis opens up a world of possibilities. If you found these facts about hydrolysis intriguing, wait until you explore the enigmatic nature of salt hydrolysis and uncover the nutritional value of maltodextrin. Dive deeper into the world of chemistry and nutrition, as you satisfy your curiosity with more captivating insights.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
