When it comes to chemical reactions and experiments, one of the key metrics that scientists and researchers strive to achieve is the percent yield. This measurement allows us to determine the efficiency and effectiveness of a reaction by comparing the amount of product obtained to the amount that should theoretically be produced. While percent yield may seem like a straightforward concept, there are many surprising factors and intricacies surrounding it.
In this article, we will delve into 14 fascinating facts about percent yield that are sure to captivate the curious minds of chemists and science enthusiasts alike. From the factors influencing percent yield to real-world applications and practical tips for improving it, we will explore the nuances of this crucial aspect of chemical reactions. So, let’s dive in and uncover some unexpected insights about percent yield!
Key Takeaways:
- Percent yield measures how well a chemical reaction works. It helps chemists make better reactions and save money in industries. Understanding it is super important for making cool new stuff!
- Percent yield is like a chemistry efficiency score. It helps us make better medicines, products, and even helps the environment. Chemists are always working to make it even better!
What is Percent Yield?
Percent yield refers to the measure of efficiency in a chemical reaction or process. It is the ratio of the actual yield to the theoretical yield, expressed as a percentage. Theoretical yield is the maximum amount of product that can be obtained based on stoichiometry, while the actual yield is the amount of product actually obtained in a laboratory or industrial setting.
The Importance of Percent Yield
Percent yield is crucial in determining the efficiency and reliability of chemical reactions. It helps chemists evaluate the effectiveness of their procedures, identify potential sources of error, and make adjustments to improve the reaction’s productivity. In industrial applications, percent yield is a key factor in optimizing production processes and ensuring cost-effectiveness.
Factors Affecting Percent Yield
Several factors can influence the percent yield of a reaction. These include the purity of the reactants, reaction conditions such as temperature and pressure, the presence of catalysts, and the extent of side reactions or competing processes. Understanding these factors is essential for achieving higher percent yields in chemical synthesis.
Percent Yield and Limiting Reactant
The concept of the limiting reactant is closely related to percent yield. In a chemical reaction, the limiting reactant is the substance that is completely consumed and determines the maximum amount of product that can be obtained. The percent yield is calculated based on the limiting reactant, as the theoretical yield is dependent on its availability.
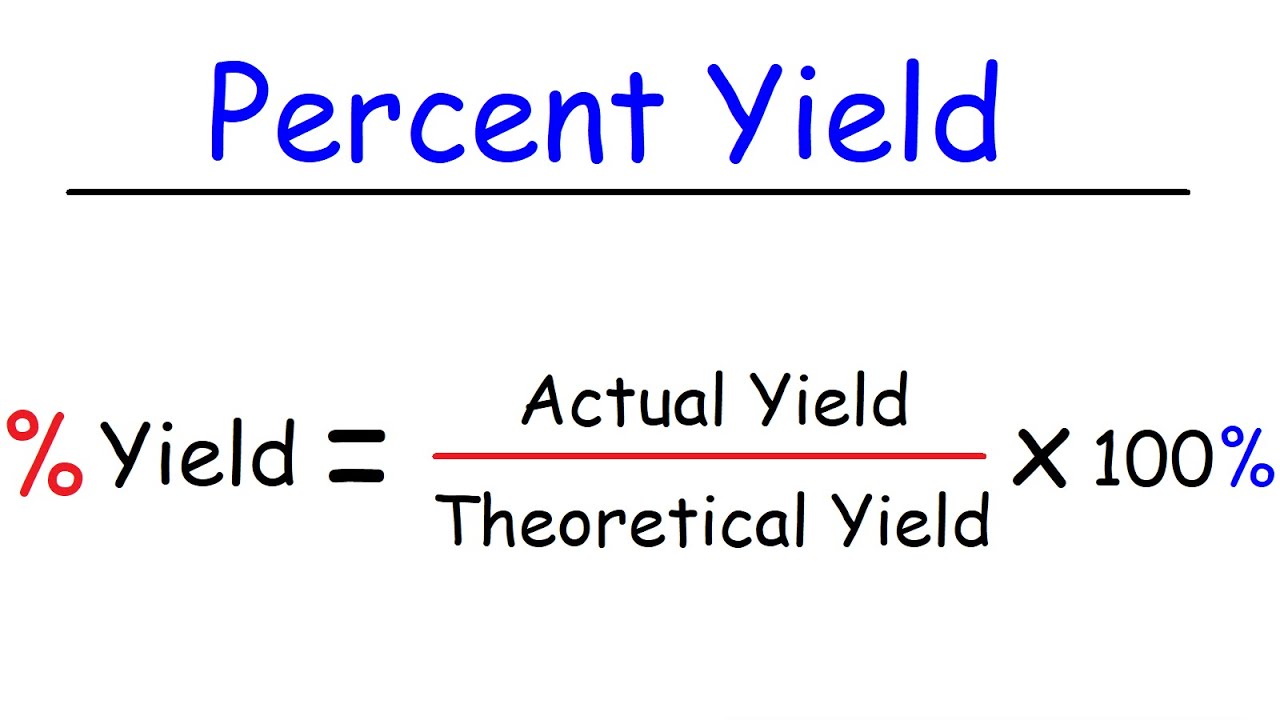
Calculating Percent Yield
To calculate percent yield, you divide the actual yield by the theoretical yield and multiply by The formula is: Percent Yield = (Actual Yield / Theoretical Yield) x This calculation allows chemists to assess the efficiency of their reactions and make improvements if necessary.
Theoretical Yield vs. Actual Yield
Theoretical yield is the amount of product predicted by stoichiometric calculations based on the quantities of reactants used. It assumes that the reaction proceeds perfectly without any side reactions or losses. Actual yield, on the other hand, refers to the amount of product obtained in a real-world scenario, which may be lower than the theoretical yield due to various factors.
Percent Yield and Stoichiometry
Percent yield is closely related to stoichiometry, which is the quantitative relationship between the reactants and products in a chemical reaction. By understanding stoichiometry, chemists can predict the theoretical yield and compare it to the actual yield, providing insights into reaction efficiency.
Real-World Applications of Percent Yield
Percent yield is utilized in various industries, including pharmaceuticals, manufacturing, and agriculture. It helps in determining the efficiency of drug synthesis, evaluating the performance of chemical processes, and optimizing agricultural practices for maximum crop yield.
Challenges in Achieving High Percent Yield
Obtaining a high percent yield can be challenging due to factors such as side reactions, incomplete reactions, or losses during purification and isolation. Chemists continually strive to improve reaction conditions and techniques to overcome these challenges and increase the efficiency of chemical processes.
Percent Yield and Environmental Sustainability
Percent yield plays a role in promoting environmental sustainability in the chemical industry. By maximizing the efficiency of reactions and minimizing wastage, it helps reduce the consumption of resources and minimize the generation of harmful by-products, leading to a more sustainable and eco-friendly approach to chemical synthesis.
Percent Yield and Quality Control
In manufacturing industries, percent yield is an essential parameter for quality control. It allows companies to monitor and ensure the consistency and reliability of their production processes, enabling them to deliver high-quality products to consumers.
Percent Yield and Economic Impact
Efficient chemical reactions with high percent yields have a significant economic impact. By maximizing the production of desired products and minimizing waste, companies can reduce costs, improve profitability, and enhance competitiveness in the market.
Continuous Improvement in Percent Yield
Chemists and researchers are constantly striving for advancements in reaction conditions, catalysts, and purification techniques to enhance percent yield. This continuous improvement leads to more efficient processes, reduced resource consumption, and increased sustainability in the field of chemistry.
Percent Yield and Future Innovations
Percent yield remains a fundamental concept in chemistry and will continue to play a vital role in future innovations. As researchers explore new synthetic methodologies and develop novel reactions, percent yield will guide their efforts to maximize efficiency and productivity.
Conclusion
In conclusion, percent yield is a crucial concept in chemistry that measures the efficiency of a chemical reaction. It allows us to determine how much product is actually obtained compared to the maximum possible amount that could be produced. Understanding percent yield not only provides insight into the efficiency of a reaction but also helps in optimizing reaction conditions and minimizing waste.
Through this article, we have explored various surprising facts about percent yield. We have learned about the factors affecting percent yield, such as impurities, side reactions, and incomplete reactions. We have also discovered how to calculate percent yield and its significance in various real-life applications.
Remember, achieving a high percent yield is not always possible, but understanding the reasons behind it can lead to improvements in future experiments and processes. So, keep experimenting, analyzing, and striving for optimum results!
FAQs
1. What is percent yield in chemistry?
Percent yield is a measurement that compares the amount of product obtained from a chemical reaction to the theoretical yield, which is the maximum amount of product that could be obtained if the reaction proceeded perfectly.
2. How is percent yield calculated?
Percent yield is calculated by dividing the actual yield (the measured amount of product obtained) by the theoretical yield and multiplying by 100. The formula is: percent yield = (actual yield / theoretical yield) x 100.
3. What factors can affect percent yield?
Several factors can affect percent yield, including impurities in reactants, side reactions that produce unwanted products, incomplete reactions, and experimental errors in measurements or techniques.
4. Why is percent yield important?
Percent yield is important as it provides insight into the efficiency of a reaction and helps in evaluating the success of a chemical synthesis or process. It allows chemists to optimize reaction conditions, minimize waste, and make improvements in their experimental procedures.
5. Can percent yield be greater than 100%?
No, percent yield cannot be greater than 100%. A percent yield greater than 100% would indicate that more product was obtained than theoretically possible, which violates the principles of conservation of mass.
Percent yield is a crucial concept in chemistry, influencing reactions and outcomes. Mastering its calculation and understanding the factors that affect it can help optimize chemical processes. Exploring real-world applications and the challenges in achieving high percent yield provides valuable insights into this fascinating topic. For those curious about the intricacies of chemical equations and relationships, our article on the enigmatic facts about stoichiometry is a must-read. Unraveling the mysteries behind these fundamental concepts will deepen your appreciation for the complex world of chemistry and its impact on our lives.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
