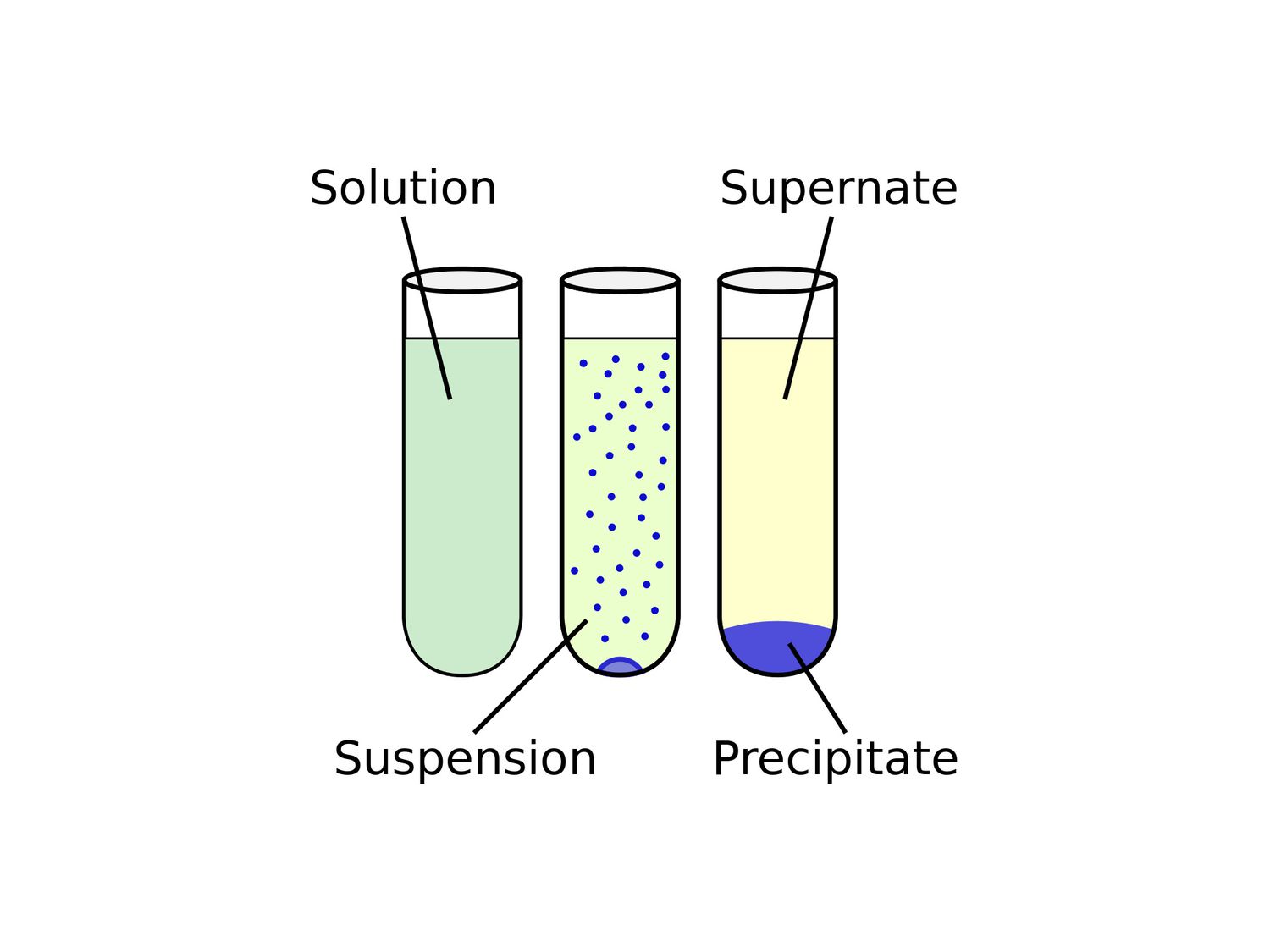
Precipitate is a fascinating concept in chemistry that often sparks curiosity and amazement. It refers to the formation of solid particles, known as precipitates, from a liquid solution. While precipitates may seem like simple chemical reactions, they hold a world of intriguing facts and phenomena waiting to be explored.
In this article, we will delve into the realm of precipitate and uncover 11 unbelievable facts that will boggle your mind. From the mesmerizing colors of precipitates to their role in environmental processes, each fact will provide a glimpse into the intricate world of chemistry. So, get ready to be astonished as we take a deep dive into the captivating world of precipitate.
Key Takeaways:
- Precipitate is the solid particles formed in a chemical reaction, and it can have different shapes and colors, play a role in determining substances, and even be encountered in everyday life.
- The formation of precipitate can indicate chemical reactions, have industrial applications, and support scientific investigations, making it a fascinating and important aspect of chemistry.
The Formation of Precipitate is a Chemical Phenomenon
Precipitate refers to the solid particles that form during a chemical reaction. It occurs when two substances react and create a new solid product that is insoluble in the reaction mixture. This fascinating chemical phenomenon has been the subject of countless experiments and research studies.
Precipitate Can Take Various Forms
One of the remarkable things about precipitate is its ability to form in various shapes and sizes. It can range from fine powders to crystalline structures, and even amorphous solids. The diversity of precipitate morphology adds to the intrigue and complexity of this chemical process.
Precipitate Plays a Crucial Role in Analytical Chemistry
In the field of analytical chemistry, precipitate plays a vital role in determining the presence and concentration of certain substances. By adding specific reagents to a sample solution, scientists can induce the formation of precipitate, which can then be filtered, dried, and weighed to determine the quantity of the desired compound.
Some Precipitates Have Unique Colors
While precipitate is commonly associated with a white or off-white color, there are instances where it exhibits unique and vibrant colors. Some precipitates can be blue, green, red, or even multi-colored due to the presence of certain metal ions or complex compounds.
Precipitate Can Be Both Permanent and Temporary
Not all precipitates are permanent. Some may dissolve back into the solution upon further addition of reagents or changes in temperature. These reversible precipitates are known as temporary precipitates and provide valuable insights into the solubility and stability of different compounds.
Factors Affecting Precipitate Formation
The formation of precipitate is influenced by several factors, including temperature, concentration, pH, and reaction kinetics. These variables can significantly impact the rate of formation, size, and characteristics of the resulting precipitate.
Precipitate Can Indicate Chemical Reactions
Precipitate formation often serves as a visual indicator of a chemical reaction taking place. It provides tangible evidence of the transformation occurring between the reactants and can help chemists identify and confirm the occurrence of specific reactions.
Precipitate Can Have Industrial Applications
Precipitate plays a crucial role in various industrial processes. For example, in wastewater treatment, precipitate formation helps remove pollutants and impurities from the water, making it safer for disposal or recycling. Precipitate is also used in the production of pigments, ceramics, and pharmaceuticals.
Precipitate Is Not Limited to Liquids
Precipitate can also form in non-liquid environments. For instance, in atmospheric chemistry, solid particles can form in the air due to the reaction of gases or aerosols. These airborne precipitates, also known as atmospheric particulate matter, can have significant implications for air quality and human health.
Precipitate Can Support Scientific Investigations
The study of precipitate offers valuable insights into the fundamental principles of chemistry and the behavior of different substances. By observing and analyzing the formation of precipitate, scientists can gain a deeper understanding of the properties, reactions, and interactions of various compounds.
Precipitate Can Be Encountered in Everyday Life
While often explored in scientific settings, precipitate can also be encountered in our daily lives. The formation of scale in kettles or the settling of sediments in certain beverages are examples of precipitate formation on a smaller scale, reminding us of the presence of chemical processes even in our routine activities.
Conclusion
Precipitation is a fascinating and essential process in the world of chemistry. Through the many unbelievable facts about precipitates that we have explored, it is clear that these solid particles play a significant role in various chemical reactions and phenomena.
From their ability to form beautiful patterns, to their importance in environmental and industrial processes, precipitates showcase the intricate nature of chemistry. Whether it’s the stunning colors they exhibit or their impact on the overall chemistry of a solution, precipitates never fail to intrigue scientists and researchers.
Understanding the properties and characteristics of precipitates allows us to delve deeper into the molecular world and uncover new insights into how substances interact. By continuing to study and explore the fascinating realm of precipitates, we can broaden our knowledge and make advancements in fields such as materials science, pharmaceuticals, and environmental chemistry.
So, the next time you observe a precipitate forming in a chemical reaction, remember to appreciate the remarkable processes taking place on a microscopic level. The world of precipitates holds many surprises and secrets yet to be revealed, and it is through curiosity and exploration that we can uncover them.
FAQs
Q: What is a precipitate?
A: A precipitate is a solid substance that forms during a chemical reaction when two liquids or solutions combine. It appears as a finely divided solid suspended in a liquid or as a solid settling at the bottom of a solution.
Q: How do precipitates form?
A: Precipitates form when two solutions containing soluble compounds are mixed and a reaction occurs that yields an insoluble product. The insoluble product separates from the solution as a solid precipitate.
Q: Can precipitates have different colors?
A: Yes, precipitates can have different colors depending on the chemical compounds involved in the reaction. Some precipitates exhibit vibrant colors due to the presence of transition metals, while others may be white or colorless.
Q: What are some real-life examples of precipitates?
A: Some real-life examples of precipitates include the formation of rust on iron surfaces, the settling of sediment in a river, and the formation of snowflakes in the atmosphere. These are all instances where solid particles are created through precipitation.
Q: Are precipitates important in environmental processes?
A: Yes, precipitates play a crucial role in environmental processes. They can help remove pollutants from water through processes like coagulation and flocculation. Precipitation reactions also occur in the atmosphere, leading to the formation of rain and snow.
Precipitation's incredible facts don't stop here! Quench your thirst for knowledge with extraordinary tidbits about this captivating phenomenon. Unravel the enigmatic world of orographic rainfall and its impact on our planet. For those curious about the intricacies of chemistry, precipitation titration holds a treasure trove of fascinating insights waiting to be discovered.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
