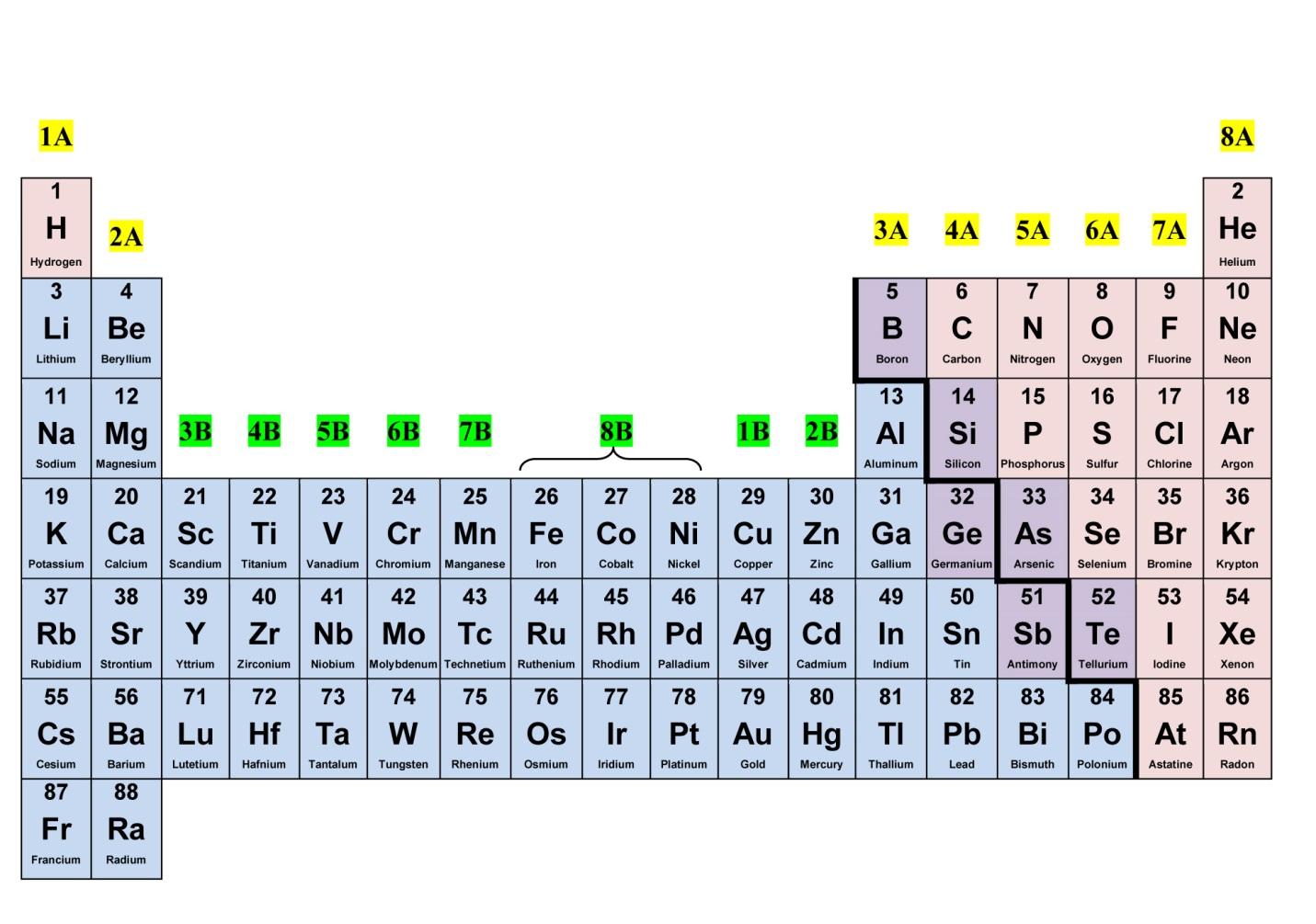
The periodic table is a fundamental tool in the world of science, serving as a visual representation of the building blocks of matter. It organizes elements based on their atomic number, electron configuration, and recurring chemical properties. As a cornerstone of chemistry and physics, the periodic table provides invaluable insights into the behavior and characteristics of elements, enabling scientists to predict their properties and reactivity.
In this article, we'll delve into 33 fascinating facts about the periodic table, shedding light on its historical significance, the arrangement of elements, and its impact on scientific advancements. From the discovery of new elements to the periodic trends that govern their behavior, we'll explore the intriguing facets of this iconic chart. Whether you're a science enthusiast, a student, or simply curious about the world around you, these facts will illuminate the captivating realm of the periodic table and its profound influence on the scientific landscape. So, let's embark on a journey through the elemental wonders encapsulated within the periodic table!
Key Takeaways:
- The Periodic Table is a fascinating tool that organizes elements and helps scientists predict their properties, fostering a deeper understanding of the building blocks of the universe.
- With its 118 known elements, the Periodic Table continues to inspire curiosity and wonder, serving as a timeless symbol of human exploration and scientific discovery.
The Periodic Table is a fundamental tool in the study of chemistry.
The Periodic Table is a comprehensive display of chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. It serves as a vital reference for scientists, educators, and students, offering a wealth of information about the building blocks of matter. Let's delve into 33 fascinating facts about the Periodic Table that showcase its significance and intrigue.
The Periodic Table was first created by Dmitri Mendeleev in 1869.
Dmitri Mendeleev, a Russian chemist, is credited with developing the first widely recognized Periodic Table. His innovative arrangement of elements based on their properties and atomic weights laid the foundation for the modern Periodic Table.
The Periodic Table organizes elements into groups and periods.
Elements in the Periodic Table are arranged into groups (columns) and periods (rows). This systematic organization allows for the classification of elements with similar characteristics and behaviors, providing valuable insights into their properties and reactivity.
There are currently 118 known elements in the Periodic Table.
As of now, the Periodic Table encompasses 118 confirmed elements, ranging from hydrogen (with atomic number 1) to oganesson (with atomic number 118). Each element contributes to the rich tapestry of chemical diversity.
The Periodic Table is a visual representation of the building blocks of the universe.
By showcasing the elements and their unique attributes, the Periodic Table offers a snapshot of the fundamental components that form all matter in the cosmos, from the air we breathe to the stars in the night sky.
The arrangement of elements in the Periodic Table reflects their atomic structure.
The layout of the Periodic Table is designed to highlight recurring patterns in the atomic structure of elements, shedding light on their electron configurations and bonding behaviors.
The Periodic Table is a powerful predictive tool.
Chemists and researchers utilize the Periodic Table to forecast the properties and behaviors of undiscovered elements, enabling them to make informed hypotheses and guide their explorations of new frontiers in chemistry.
The Periodic Table features various element categories.
Elements in the Periodic Table are categorized as metals, nonmetals, and metalloids, each with distinct characteristics and applications in numerous scientific and industrial fields.
The Periodic Table's organization reveals trends in element properties.
By examining the Periodic Table, one can discern patterns in properties such as atomic radius, electronegativity, and ionization energy, providing valuable insights into the behavior of elements within specific groups and periods.
The Periodic Table has undergone significant evolution.
Since its inception, the Periodic Table has evolved through the discovery of new elements and advancements in scientific understanding, leading to refinements in its organization and the inclusion of additional elements.
The Periodic Table accommodates synthetic elements.
In addition to naturally occurring elements, the Periodic Table incorporates synthetic elements that have been created through laboratory processes, expanding our understanding of atomic structure and the potential applications of these elements.
The Periodic Table fosters a deeper comprehension of chemical bonding.
By showcasing elements with similar properties in close proximity, the Periodic Table aids in elucidating the principles of chemical bonding and the formation of compounds, enriching our grasp of chemical interactions.
The Periodic Table highlights the prevalence of certain elements in Earth's crust.
Certain elements, such as oxygen, silicon, and aluminum, are abundant in Earth's crust, a fact that the Periodic Table underscores, showcasing the elemental composition of our planet.
The Periodic Table underscores the diversity of elements in the universe.
From the inert noble gases to the highly reactive alkali metals, the Periodic Table showcases the vast array of elements that contribute to the complexity and richness of the natural world.
The Periodic Table serves as a foundation for understanding chemical reactions.
By elucidating the properties and behaviors of elements, the Periodic Table forms the basis for comprehending chemical reactions and the transformations of matter, a cornerstone of chemistry.
The Periodic Table is a valuable educational resource.
In classrooms and laboratories worldwide, the Periodic Table serves as an indispensable educational tool, aiding students in grasping the properties and relationships of elements as they embark on their scientific journeys.
The Periodic Table has inspired artistic and cultural interpretations.
Beyond its scientific significance, the Periodic Table has sparked artistic and cultural expressions, serving as a muse for creative works that celebrate the beauty and order inherent in the arrangement of elements.
The Periodic Table continues to captivate and intrigue scientists and enthusiasts alike.
With its enduring relevance and ongoing contributions to scientific endeavors, the Periodic Table maintains its allure, drawing the fascination of those who seek to unravel the mysteries of the elements.
The Periodic Table fosters interdisciplinary connections.
Its comprehensive depiction of elements and their properties facilitates interdisciplinary collaborations, bridging the realms of chemistry, physics, materials science, and beyond, to foster new innovations and discoveries.
The Periodic Table is celebrated through periodic table days and events.
Across the globe, periodic table-themed observances and events honor the significance of this foundational tool, uniting individuals in their appreciation for the Periodic Table's role in advancing scientific knowledge.
The Periodic Table fuels advancements in technology and industry.
By elucidating the properties and potential applications of elements, the Periodic Table underpins innovations in technology, materials science, and industrial processes, driving progress and shaping the modern world.
The Periodic Table showcases the beauty of scientific organization.
Its elegant arrangement of elements reflects the harmony and order inherent in the natural world, offering a testament to the beauty of scientific organization and the wonders of the universe.
The Periodic Table embodies the collaborative nature of scientific discovery.
The development and refinement of the Periodic Table stand as a testament to the collaborative efforts of scientists across generations, underscoring the collective pursuit of knowledge and understanding in the scientific community.
The Periodic Table serves as a gateway to exploration and inquiry.
With its wealth of information and avenues for discovery, the Periodic Table inspires curiosity and serves as a springboard for further exploration into the properties and behaviors of elements, perpetuating the spirit of scientific inquiry.
The Periodic Table transcends cultural and linguistic boundaries.
Its universal language of elements and their properties transcends cultural and linguistic barriers, serving as a unifying tool in the global pursuit of scientific knowledge and understanding.
The Periodic Table underscores the interconnectedness of the natural world.
By showcasing the elements that form the basis of all matter, the Periodic Table emphasizes the interconnectedness of the natural world, fostering an appreciation for the unity and diversity of the elements around us.
The Periodic Table continues to evolve with scientific advancements.
As scientific knowledge expands and new discoveries unfold, the Periodic Table remains a dynamic and evolving resource, adapting to incorporate the latest insights and revelations in the realm of chemistry.
The Periodic Table inspires the next generation of scientists and innovators.
Through its captivating portrayal of the elements and their properties, the Periodic Table ignites the curiosity and passion of aspiring scientists, nurturing the future leaders and trailblazers in the field of chemistry and beyond.
The Periodic Table exemplifies the power of human ingenuity and exploration.
Its creation and ongoing refinement stand as a testament to human ingenuity and the relentless pursuit of understanding, showcasing the remarkable achievements of scientific exploration and discovery.
The Periodic Table embodies the essence of scientific discovery and enlightenment.
In its elegant organization of elements and their properties, the Periodic Table encapsulates the spirit of scientific inquiry and enlightenment, serving as a timeless symbol of humanity's quest to unravel the mysteries of the natural world.
The Periodic Table is a cornerstone of chemical knowledge and understanding.
With its wealth of information and insights, the Periodic Table remains a cornerstone of chemical knowledge, guiding researchers, educators, and enthusiasts in their explorations and interpretations of the elements and their myriad applications.
The Periodic Table continues to inspire wonder and curiosity.
From its inception to the present day, the Periodic Table continues to inspire wonder and curiosity, captivating the imagination and fueling the passion of those who seek to unravel the secrets of the elements and their profound significance in the universe.
The Periodic Table serves as a testament to the enduring legacy of scientific exploration and discovery.
As a testament to human curiosity and the relentless pursuit of knowledge, the Periodic Table stands as an enduring legacy of scientific exploration and discovery, offering a timeless testament to the indomitable spirit of scientific inquiry.
The Periodic Table stands as a testament to the enduring legacy of scientific exploration and discovery, serving as a timeless symbol of humanity's quest to unravel the mysteries of the natural world. With its captivating portrayal of the elements and their properties, the Periodic Table continues to inspire wonder and curiosity, fueling the passion of those who seek to unravel the secrets of the elements and their profound significance in the universe.
Conclusion
In conclusion, the periodic table is not just a chart of elements; it is a testament to the incredible diversity and complexity of the natural world. Each element has its own unique properties and significance, contributing to the vast tapestry of chemistry and the universe as a whole. By delving into the fascinating facts and stories behind the periodic table, we gain a deeper appreciation for the building blocks of matter and the scientific achievements that have shaped our understanding of the world around us.
FAQs
What is the significance of the periodic table?The periodic table is a fundamental tool in chemistry, organizing elements based on their atomic structure and properties. It provides a systematic way to understand the behavior and relationships of elements, laying the groundwork for countless scientific discoveries and technological advancements.
How many elements are there in the periodic table?Currently, there are 118 confirmed elements in the periodic table, with each one playing a unique role in the composition of matter and the functioning of the natural world.
The periodic table holds countless secrets waiting to be explored. From the enigmatic properties of its elements to the captivating stories behind their discovery, this fundamental scientific tool never ceases to amaze. If you're hungry for more periodic table knowledge, our articles delve into the mysteries surrounding this iconic chart. Uncover the hidden depths of the periodic table's organization, learn about the fascinating characteristics of specific elements like europium, and embark on a journey through the periodic table's rich history. Satisfy your curiosity and expand your understanding of chemistry with these engaging reads.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
