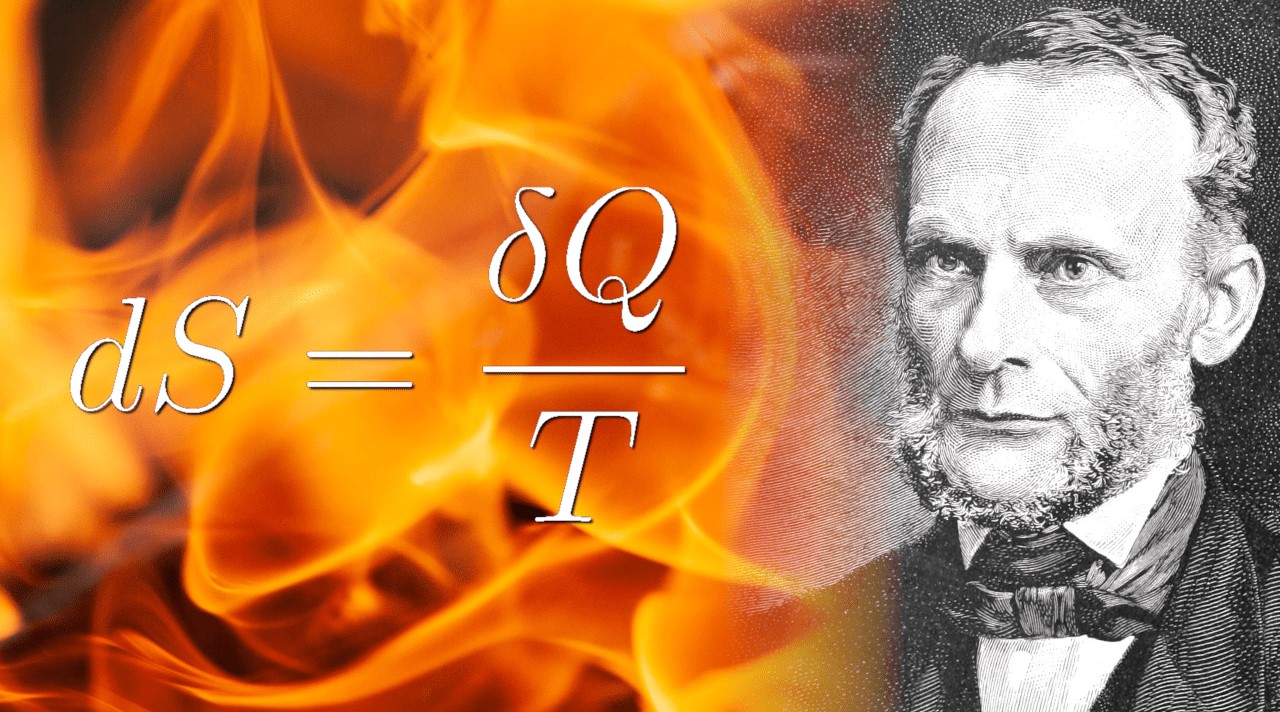
Rudolf Clausius, the renowned physicist and mathematician, is a name that is synonymous with the fundamental laws of thermodynamics. His contributions to the field have had a profound impact on our understanding of energy, heat, and entropy. While his name may not be as well-known as some other famous scientists, the life and work of Clausius are nothing short of fascinating.
In this article, we will uncover 19 unbelievable facts about Rudolf Clausius that will shed light on both his personal and professional life. From his groundbreaking discoveries to his struggles and triumphs, Clausius’ story is a testament to the power of dedication and innovation in the world of science. So, let’s dive in and unravel the incredible journey of this exceptional scientist.
Key Takeaways:
- Rudolf Clausius, a renowned physicist, introduced the concept of entropy and formulated the second law of thermodynamics, shaping our understanding of energy and matter.
- His groundbreaking work on thermodynamics and entropy continues to inspire scientists and find applications in engineering, chemistry, and material science.
Rudolf Clausius was a renowned German physicist.
Rudolf Clausius, born on January 2, 1822, in Poland, was a highly influential physicist known for his significant contributions to the field of thermodynamics.
He formulated the second law of thermodynamics.
Clausius is widely recognized for his formulation of the second law of thermodynamics, which states that the entropy of an isolated system tends to increase over time.
Clausius introduced the concept of entropy.
One of Clausius’s major achievements was introducing the concept of entropy, a measure of the disorder or randomness in a system.
He developed the theory of heat engines.
Clausius extensively studied heat engines and formulated the mathematical theory behind their operation, laying the foundation for the field of thermodynamics.
Clausius made significant advancements in kinetic theory.
His work on kinetic theory revolutionized the understanding of gas molecules‘ behavior and their relationship to temperature and pressure.
He derived the Clausius-Clapeyron equation.
The Clausius-Clapeyron equation, developed by Clausius in collaboration with French engineer Émile Clapeyron, relates the vapor pressure of a substance to its temperature.
Clausius introduced the concept of reversible and irreversible processes.
He classified thermodynamic processes as either reversible or irreversible, based on their ability to be reversed without any loss or gain of energy.
He proposed the mathematical expression for entropy change.
Clausius formulated the mathematical expression for calculating the change in entropy during a thermodynamic process, providing a quantitative measure of the system’s disorder.
Clausius laid the foundation for the study of equilibrium in thermodynamics.
His work in defining and understanding thermal equilibrium helped establish the principles necessary for the study of thermodynamic systems at rest.
He was a professor at the Royal Artillery and Engineering School.
Clausius served as a professor at the Royal Artillery and Engineering School in Berlin, where he taught various subjects including mechanics and mathematical physics.
Clausius was the first to use the term “entropy.”
In his publication “Über die bewegende Kraft der Wärme” (On the Moving Force of Heat), Clausius coined the term “entropy” to describe the energy that cannot be converted into useful work.
He published over 300 scientific papers.
Clausius was a prolific writer and published numerous scientific papers during his career, covering a wide range of topics in physics and thermodynamics.
Clausius received several prestigious awards for his contributions.
His groundbreaking work in thermodynamics earned him numerous accolades, including the Copley Medal from the Royal Society in 1868.
He laid the foundation for the concept of entropy production.
Clausius’s research provided the groundwork for the understanding of entropy production in various natural and engineered systems.
Clausius’s principles have applications in various fields.
The principles and laws formulated by Clausius in thermodynamics find applications in fields such as engineering, chemistry, meteorology, and material science.
He developed the concept of adiabatic processes.
Clausius further contributed to thermodynamics by introducing the concept of adiabatic processes, where no heat is exchanged with the surroundings.
Clausius made important advancements in the theory of heat conduction.
His research on heat conduction led to significant advancements in understanding the transfer of thermal energy through different materials.
He played a crucial role in laying the foundation for the second law of thermodynamics.
Clausius’s work on the second law and his efforts to establish a precise mathematical expression for entropy were instrumental in shaping the development of thermodynamics.
Clausius’s legacy continues to inspire scientists and physicists.
His profound contributions to thermodynamics and his pioneering work on entropy and the second law continue to shape our understanding of the fundamental principles governing the behavior of energy and matter.
Conclusion
In conclusion, Rudolf Clausius was an exceptional figure in the field of thermodynamics and a pioneer in the study of entropy. His groundbreaking work and contributions have had a significant impact on our understanding of the laws of physics and the behavior of gases and heat. From formulating the second law of thermodynamics to developing the concept of entropy, Clausius revolutionized the field and laid the foundation for future advancements.Clausius’ unwavering commitment to scientific research and his ability to apply mathematical principles to complex phenomena made him a key figure in the development of modern thermodynamics. His ideas and theories continue to be fundamental to many aspects of physics and engineering, serving as a cornerstone for numerous scientific advancements.In summary, the 19 unbelievable facts about Rudolf Clausius highlight his immense contributions to the field of thermodynamics and his relentless pursuit of scientific knowledge. Through his work, Clausius has left an indelible mark on the scientific community and has paved the way for further exploration and understanding of the laws that govern our physical world.
FAQs
1. Who was Rudolf Clausius?
Rudolf Clausius was a renowned German mathematician and physicist who is best known for his contributions to the field of thermodynamics. He formulated the second law of thermodynamics and developed the concept of entropy.
2. What is the second law of thermodynamics?
The second law of thermodynamics states that in any natural thermodynamic process, the total entropy of a closed system will always increase over time. This law explains the irreversible nature of certain processes and the tendency towards disorder in the universe.
3. What is entropy?
Entropy is a fundamental concept in thermodynamics that quantifies the amount of disorder or randomness in a system. It is a measure of the distribution and availability of energy within a system.
4. How did Clausius contribute to thermodynamics?
Clausius made significant contributions to the field of thermodynamics by formulating the second law, which describes the direction in which heat flows between systems. He also introduced the concept of entropy to measure the overall energy distribution and predict the behavior of systems.
5. What is the significance of Clausius’ work?
Clausius’ work in thermodynamics laid the foundation for our understanding of energy transfer, heat flow, and the behavior of gases. His theories and equations have been instrumental in numerous scientific and engineering applications, making him a key figure in the development of modern thermodynamics.
Rudolf Clausius's groundbreaking work in thermodynamics continues to inspire scientists today. His development of the Clausius-Clapeyron equation revolutionized our understanding of phase transitions and equilibrium states. Clausius's formulation of the first law of thermodynamics, also known as the law of energy conservation, laid the foundation for modern physics. Explore more fascinating facts about these pivotal scientific concepts and gain a deeper appreciation for Clausius's lasting impact on the field of thermodynamics.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
