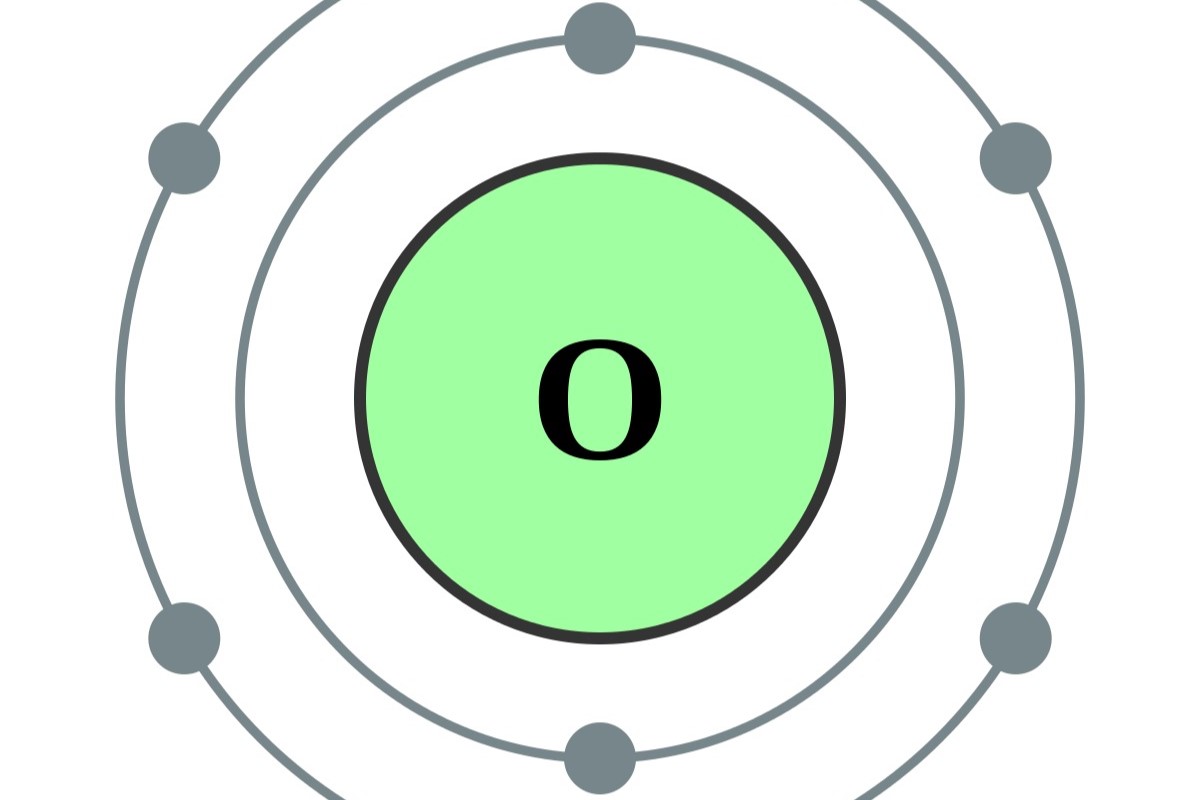
What are electron shells? Electron shells are layers around an atom's nucleus where electrons orbit. These shells determine how atoms interact with each other. Think of them as invisible highways where electrons zoom around. Each shell can hold a certain number of electrons. The first shell holds up to 2, the second up to 8, and so on. The arrangement of electrons in these shells affects an element's chemical properties. For example, elements with full outer shells are less reactive. Understanding electron shells helps explain why some elements bond easily while others don't. Ready to dive into the world of electron shells? Let's get started!
What Are Electron Shells?
Electron shells are fascinating structures surrounding an atom's nucleus. They play a crucial role in determining an element's chemical properties. Let's dive into some intriguing facts about these shells.
-
Electron shells are energy levels. Electrons orbit the nucleus in specific energy levels called shells. Each shell can hold a certain number of electrons.
-
The first shell holds two electrons. The closest shell to the nucleus, known as the K shell, can only accommodate two electrons.
-
Higher shells hold more electrons. As you move away from the nucleus, each subsequent shell can hold more electrons. For example, the second shell (L shell) can hold eight electrons.
How Electron Shells Affect Chemical Behavior
The arrangement of electrons in shells influences how atoms interact with each other. This interaction forms the basis of chemistry.
-
Valence electrons determine reactivity. The electrons in the outermost shell, called valence electrons, are crucial in chemical reactions. Atoms with incomplete outer shells tend to be more reactive.
-
Noble gases have full outer shells. Elements like helium, neon, and argon have complete outer shells, making them very stable and unreactive.
-
Ionic bonds form through electron transfer. Atoms can achieve full outer shells by transferring electrons, resulting in ionic bonds. For example, sodium donates an electron to chlorine, forming table salt.
Electron Shells and the Periodic Table
The periodic table is organized based on electron configurations, which are directly related to electron shells.
-
Periods represent energy levels. Each row (period) in the periodic table corresponds to a different electron shell. Elements in the same period have electrons in the same energy level.
-
Groups share similar properties. Elements in the same column (group) have similar valence electron configurations, leading to similar chemical behaviors.
-
Transition metals have complex electron arrangements. These elements, found in the middle of the periodic table, have electrons filling inner shells, leading to unique properties.
Quantum Mechanics and Electron Shells
Quantum mechanics provides a deeper understanding of electron shells and their behavior.
-
Electrons exist in orbitals. Within each shell, electrons occupy specific regions called orbitals. Each orbital can hold up to two electrons.
-
Pauli exclusion principle. This principle states that no two electrons in an atom can have the same set of quantum numbers, ensuring unique electron configurations.
-
Hund's rule. Electrons fill orbitals in a way that maximizes the number of unpaired electrons, leading to more stable configurations.
Practical Applications of Electron Shells
Understanding electron shells has practical implications in various fields, from chemistry to technology.
-
Semiconductors rely on electron behavior. The properties of semiconductors, used in electronics, depend on the arrangement of electrons in their shells.
-
Spectroscopy reveals electron transitions. This technique studies the interaction of light with matter, providing insights into electron transitions between shells.
Final Thoughts on Electron Shells
Electron shells are more than just a part of an atom's structure. They play a crucial role in determining how elements interact with each other. Understanding these shells helps explain why certain elements bond together while others don't. Electrons in the outermost shell, or valence electrons, are especially important in chemical reactions. Knowing about electron shells can also shed light on the periodic table's layout. Each row corresponds to a different shell being filled with electrons. This knowledge isn't just for scientists; it can help anyone grasp the basics of chemistry. Whether you're a student, a teacher, or just curious, understanding electron shells can make the world of atoms a bit less mysterious. So next time you look at the periodic table, remember the hidden layers of electron shells that make it all work.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.