Noncompetitive inhibition is a fascinating topic in biochemistry that affects how enzymes work. Unlike competitive inhibition, where inhibitors compete with substrates for the active site, noncompetitive inhibitors bind to a different part of the enzyme. This binding changes the enzyme's shape, making it less effective or even inactive. Why does this matter? Understanding noncompetitive inhibition helps in drug development, disease treatment, and even agriculture. It’s like having a key that fits into a lock but changes the lock’s mechanism, making it impossible for the original key to work. Ready to dive into 39 intriguing facts about noncompetitive inhibition? Let's get started!
What is Noncompetitive Inhibition?
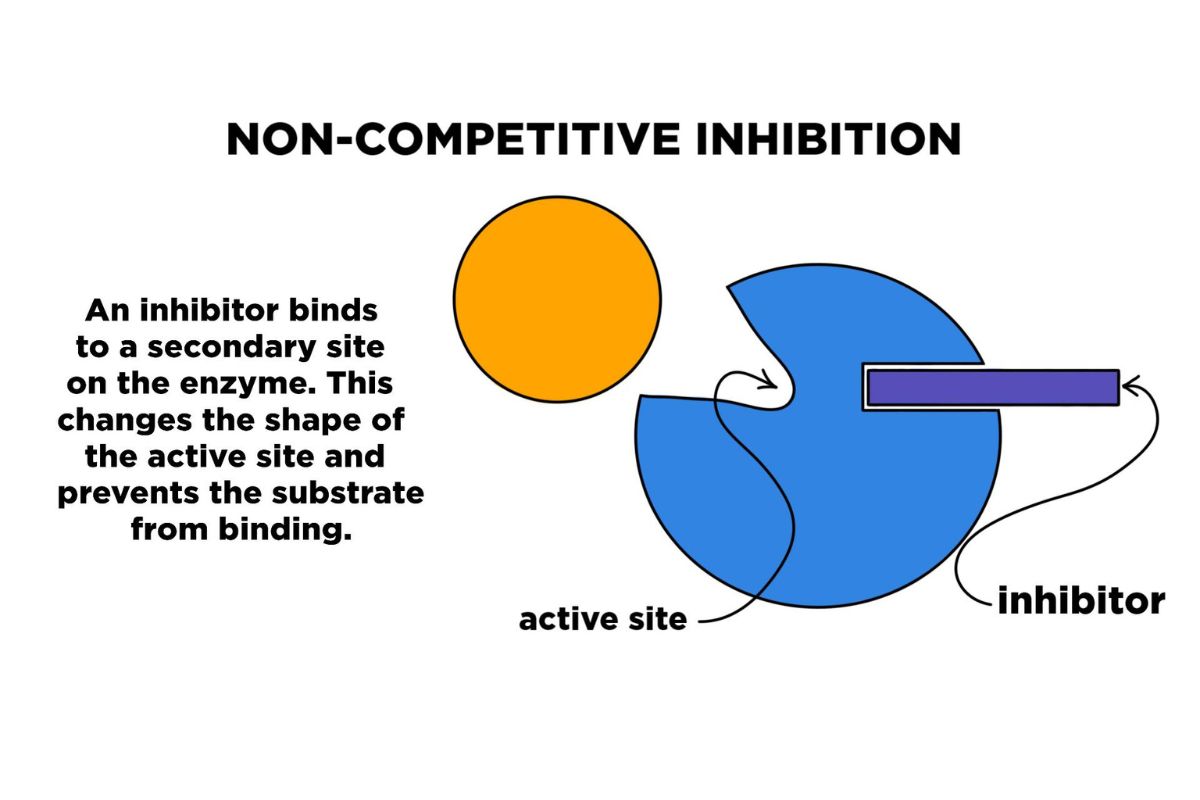
Noncompetitive inhibition is a type of enzyme inhibition where an inhibitor reduces the activity of an enzyme without binding to the active site. This process is crucial in regulating metabolic pathways and can have significant implications in medicine and biochemistry.
- Noncompetitive inhibitors bind to an enzyme at a site other than the active site, known as the allosteric site.
- These inhibitors change the enzyme's shape, making it less effective or completely inactive.
- Unlike competitive inhibitors, noncompetitive inhibitors do not compete with the substrate for the active site.
- Noncompetitive inhibition can be reversible or irreversible, depending on the nature of the inhibitor.
- This type of inhibition is often used by cells to regulate enzyme activity and maintain homeostasis.
How Does Noncompetitive Inhibition Work?
Understanding the mechanics of noncompetitive inhibition helps in grasping its role in biological systems. Here's a closer look at how it functions:
- When a noncompetitive inhibitor binds to the allosteric site, it induces a conformational change in the enzyme.
- This change can alter the shape of the active site, preventing the substrate from binding effectively.
- Even if the substrate binds, the enzyme's catalytic activity is reduced or halted.
- Noncompetitive inhibition affects the maximum reaction rate (Vmax) but does not change the substrate concentration needed to reach half of Vmax (Km).
- This type of inhibition is often depicted in enzyme kinetics graphs as a decrease in Vmax with no change in Km.
Examples of Noncompetitive Inhibition
Noncompetitive inhibition is not just a theoretical concept; it has practical examples in real-world biology and medicine.
- Heavy metals like lead and mercury act as noncompetitive inhibitors for various enzymes.
- Certain antibiotics, such as tetracyclines, inhibit bacterial enzymes noncompetitively.
- The drug allopurinol, used to treat gout, noncompetitively inhibits xanthine oxidase.
- Cyanide is a potent noncompetitive inhibitor of cytochrome c oxidase in the electron transport chain.
- Some pesticides work by noncompetitively inhibiting enzymes essential for insect survival.
Importance in Metabolic Pathways
Noncompetitive inhibition plays a vital role in regulating metabolic pathways, ensuring that cells function efficiently.
- It helps in feedback inhibition, where the end product of a pathway inhibits an enzyme involved earlier in the pathway.
- This regulation prevents the overproduction of substances within the cell.
- Noncompetitive inhibition allows for fine-tuning of enzyme activity in response to changing cellular conditions.
- It can help cells adapt to environmental stress by modulating enzyme functions.
- This type of inhibition is crucial for maintaining metabolic balance and energy efficiency.
Noncompetitive Inhibition in Drug Design
Pharmaceutical research often leverages noncompetitive inhibition to develop new drugs and therapies.
- Noncompetitive inhibitors can be designed to target specific enzymes involved in disease processes.
- These inhibitors are less likely to be outcompeted by high substrate concentrations, making them effective even in varying conditions.
- Drugs that act as noncompetitive inhibitors can provide more consistent therapeutic effects.
- This approach can be used to develop treatments for diseases like cancer, where enzyme regulation is disrupted.
- Noncompetitive inhibitors can also be used to combat antibiotic resistance by targeting bacterial enzymes.
Challenges and Considerations
While noncompetitive inhibition offers many benefits, it also presents certain challenges and considerations.
- Designing noncompetitive inhibitors requires a deep understanding of enzyme structure and function.
- These inhibitors must be specific to avoid off-target effects that could harm healthy cells.
- Noncompetitive inhibition can sometimes lead to unintended side effects if not carefully controlled.
- Researchers must consider the potential for resistance development, especially in microbial targets.
- The balance between efficacy and safety is crucial in developing noncompetitive inhibitors for therapeutic use.
Future Directions in Noncompetitive Inhibition Research
The study of noncompetitive inhibition continues to evolve, with new discoveries and applications emerging.
- Advances in structural biology are providing detailed insights into enzyme-inhibitor interactions.
- Computational modeling is helping predict the effects of potential noncompetitive inhibitors.
- Researchers are exploring the use of noncompetitive inhibition in personalized medicine.
- New techniques in drug delivery are enhancing the effectiveness of noncompetitive inhibitors.
- The development of more selective and potent noncompetitive inhibitors is a key focus in pharmaceutical research.
Summary of Key Points
Noncompetitive inhibition is a complex but essential concept in biochemistry and medicine. Here are some key takeaways:
- It involves inhibitors binding to an allosteric site, not the active site.
- This type of inhibition changes the enzyme's shape and reduces its activity.
- Noncompetitive inhibition is crucial for regulating metabolic pathways and maintaining cellular balance.
- It has significant applications in drug design and therapeutic interventions.
Final Thoughts on Noncompetitive Inhibition
Noncompetitive inhibition is a fascinating process that plays a crucial role in regulating enzyme activity. Unlike competitive inhibition, where inhibitors compete with substrates for the active site, noncompetitive inhibitors bind to a different part of the enzyme. This binding changes the enzyme's shape, making it less effective at catalyzing reactions.
Understanding this mechanism helps in various fields, from drug development to biochemistry research. It offers insights into how certain medications work and how metabolic pathways are controlled. Knowing these facts can aid in designing better drugs and treatments for diseases.
So, next time you hear about enzyme inhibitors, you'll know there's more to the story than just blocking the active site. Noncompetitive inhibition adds another layer of complexity and control, making it a key concept in the world of biochemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
