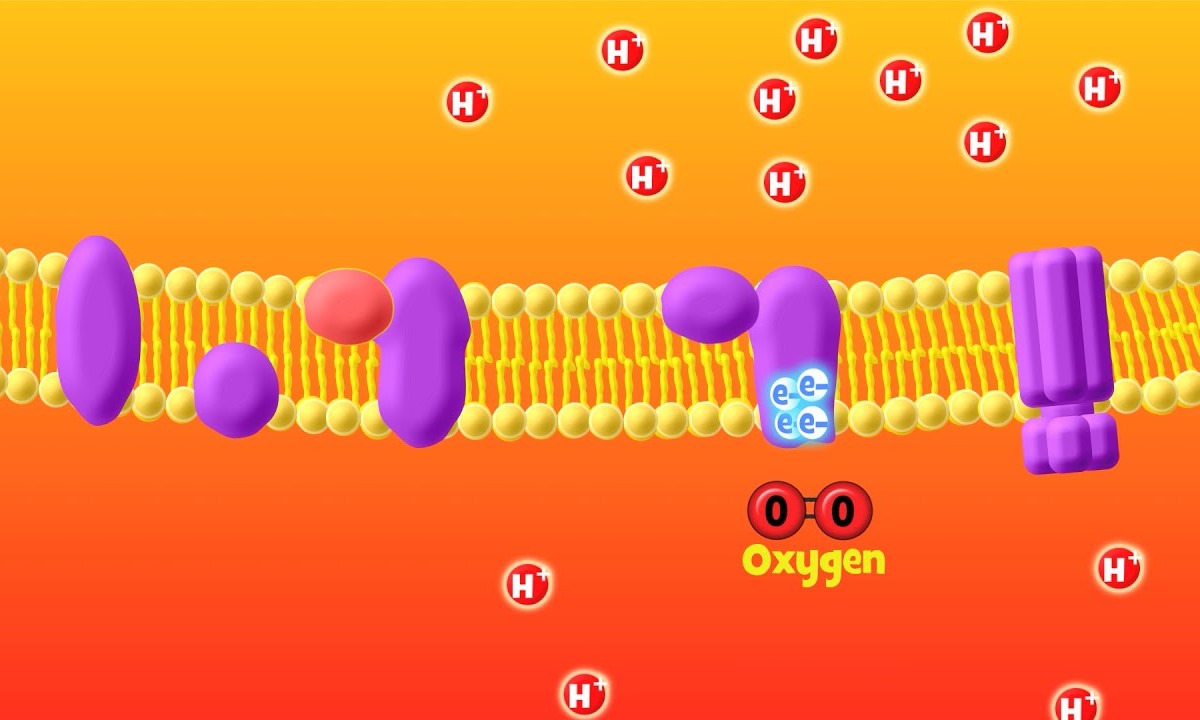
What is the electron transport chain? The electron transport chain (ETC) is a series of protein complexes and other molecules embedded in the inner mitochondrial membrane. These complexes transfer electrons from electron donors to electron acceptors via redox reactions. This process releases energy used to form ATP, the cell's main energy currency. The ETC is crucial for cellular respiration, which powers most cellular activities. Without it, cells couldn't efficiently produce energy. Understanding the ETC helps us grasp how our bodies convert food into usable energy. Let's dive into 26 intriguing facts about these vital complexes!
What is the Electron Transport Chain?
The Electron Transport Chain (ETC) is a series of protein complexes and other molecules embedded in the inner mitochondrial membrane. It plays a crucial role in cellular respiration by producing ATP, the energy currency of the cell. Here are some fascinating facts about the ETC complexes.
Complex I: NADH Dehydrogenase
Complex I is the first stop in the ETC. It receives electrons from NADH and transfers them to ubiquinone.
- Complex I is the largest of the ETC complexes, consisting of 45 subunits.
- It pumps four protons across the mitochondrial membrane for each NADH molecule oxidized.
- This complex is also known as NADH:ubiquinone oxidoreductase.
- Mutations in Complex I can lead to mitochondrial diseases like Leigh syndrome.
Complex II: Succinate Dehydrogenase
Complex II is unique because it is part of both the ETC and the citric acid cycle.
- Complex II does not pump protons across the membrane.
- It transfers electrons from succinate to ubiquinone.
- This complex is also known as succinate:ubiquinone oxidoreductase.
- It contains a heme group that helps in electron transfer.
Complex III: Cytochrome bc1 Complex
Complex III transfers electrons from ubiquinol to cytochrome c.
- Complex III pumps four protons across the membrane per electron pair.
- It is also known as ubiquinol:cytochrome c oxidoreductase.
- This complex contains two cytochromes, b and c1.
- Antimycin A is a known inhibitor of Complex III.
Complex IV: Cytochrome c Oxidase
Complex IV is the final complex in the ETC, transferring electrons to oxygen.
- Complex IV pumps two protons across the membrane per electron pair.
- It reduces oxygen to water, a crucial step in cellular respiration.
- This complex contains copper and heme groups.
- Cyanide is a potent inhibitor of Complex IV, leading to cellular asphyxiation.
Ubiquinone and Cytochrome c
These two molecules shuttle electrons between the complexes.
- Ubiquinone, also known as coenzyme Q, is lipid-soluble and moves freely within the membrane.
- Cytochrome c is a small protein that transfers electrons between Complex III and IV.
- Both ubiquinone and cytochrome c are essential for the proper functioning of the ETC.
- Ubiquinone can accept two electrons and two protons, becoming ubiquinol.
ATP Synthase: The Final Step
Though not part of the ETC, ATP synthase uses the proton gradient created by the ETC to produce ATP.
- ATP synthase is also known as Complex V.
- It produces ATP by allowing protons to flow back into the mitochondrial matrix.
- This process is known as chemiosmosis.
- ATP synthase consists of two main parts: F0 and F1.
- The F1 portion is responsible for the actual synthesis of ATP.
- ATP synthase can produce up to 34 ATP molecules per glucose molecule oxidized.
The Electron Transport Chain is a marvel of biological engineering, crucial for life as we know it.
Final Thoughts on Electron Transport Chain Complexes
Understanding the electron transport chain complexes is crucial for grasping how cells produce energy. These complexes, found in the mitochondria, play a vital role in cellular respiration. They transfer electrons through a series of reactions, ultimately producing ATP, the energy currency of the cell. Without these complexes, cells wouldn't be able to generate the energy needed for survival.
Knowing the intricacies of these complexes can help in fields like biochemistry and medicine. For instance, defects in these complexes can lead to various diseases, making them a target for medical research.
In short, the electron transport chain is a fascinating and essential part of cellular function. Whether you're a student, a researcher, or just curious, understanding these complexes offers valuable insights into how life operates at a molecular level.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.

