Radioactive decay might sound like something out of a sci-fi movie, but it's a natural process happening all around us. Ever wondered why certain elements emit radiation? Radioactive decay is the breakdown of an unstable atomic nucleus, releasing energy in the form of radiation. This process transforms the original element into a different one, sometimes even changing its chemical properties. From powering nuclear reactors to helping doctors diagnose diseases, understanding radioactive decay is crucial. But did you know it also plays a role in dating ancient artifacts and rocks? Buckle up as we dive into 37 intriguing facts about radioactive decay that will blow your mind!
What is Radioactive Decay?
Radioactive decay is a natural process where unstable atomic nuclei lose energy by emitting radiation. This phenomenon is crucial in various fields, from medicine to archaeology. Let's dive into some fascinating facts about radioactive decay.
-
Radioactive decay occurs when an unstable atomic nucleus transforms into a more stable one by releasing energy in the form of radiation.
-
There are three main types of radioactive decay: alpha, beta, and gamma decay. Each type involves different particles and energy levels.
-
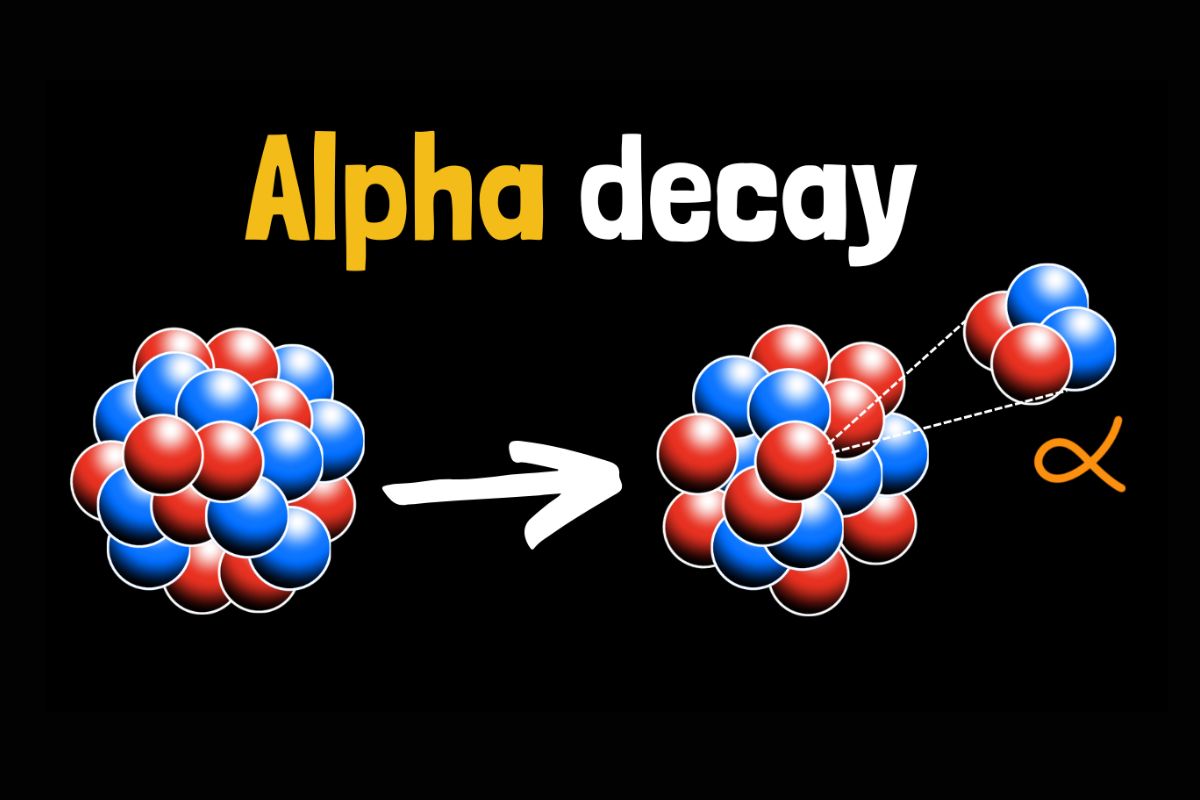
Alpha decay releases an alpha particle, which consists of two protons and two neutrons. This type of decay decreases the atomic number by two and the mass number by four.
-
Beta decay involves the transformation of a neutron into a proton or vice versa, emitting a beta particle (an electron or positron) and an antineutrino or neutrino.
-
Gamma decay emits high-energy photons called gamma rays. This type of decay usually follows alpha or beta decay to release excess energy from the nucleus.
The Role of Half-Life in Radioactive Decay
Half-life is a critical concept in understanding radioactive decay. It represents the time required for half of a radioactive substance to decay.
-
The half-life of a radioactive isotope can range from fractions of a second to billions of years, depending on the isotope.
-
Carbon-14, used in radiocarbon dating, has a half-life of about 5,730 years. This property helps scientists determine the age of ancient artifacts and fossils.
-
Uranium-238, a common isotope used in dating rocks, has a half-life of approximately 4.5 billion years, making it useful for studying the Earth's history.
-
Radon-222, a radioactive gas found in homes, has a half-life of 3.8 days. Its short half-life makes it a concern for indoor air quality.
-
The concept of half-life helps in calculating the remaining amount of a radioactive substance over time, which is essential in nuclear medicine and environmental science.
Applications of Radioactive Decay
Radioactive decay has numerous applications across various fields, from healthcare to energy production.
-
In nuclear medicine, radioactive isotopes are used for diagnostic imaging and cancer treatment. For example, iodine-131 is used to treat thyroid cancer.
-
Radiocarbon dating relies on the decay of carbon-14 to estimate the age of organic materials, such as wood and bones.
-
Smoke detectors often contain americium-241, a radioactive isotope that helps detect smoke particles through ionization.
-
Nuclear power plants use the heat generated from radioactive decay to produce electricity. Uranium-235 and plutonium-239 are common fuel sources.
-
Radioactive tracers are used in agriculture to study nutrient uptake in plants and improve crop yields.
Safety and Risks of Radioactive Decay
While radioactive decay has many benefits, it also poses risks that require careful management.
-
Exposure to high levels of radiation can cause acute radiation sickness, characterized by nausea, vomiting, and fatigue.
-
Long-term exposure to low levels of radiation increases the risk of cancer, particularly leukemia and thyroid cancer.
-
Radon gas, a byproduct of uranium decay, is the second leading cause of lung cancer after smoking.
-
Proper disposal of radioactive waste is crucial to prevent environmental contamination and protect public health.
-
Lead shielding and distance are effective methods to protect against radiation exposure in medical and industrial settings.
Interesting Facts About Radioactive Elements
Radioactive elements have unique properties and histories that make them fascinating to study.
-
Marie Curie, a pioneering scientist, discovered the radioactive elements polonium and radium in the late 19th century.
-
Uranium, the heaviest naturally occurring element, is named after the planet Uranus.
-
Plutonium, used in nuclear weapons and reactors, is named after the dwarf planet Pluto.
-
Technetium, the first artificially produced element, is used in medical imaging to diagnose heart disease and cancer.
-
Francium, one of the rarest elements on Earth, is highly radioactive and has a half-life of just 22 minutes.
Environmental Impact of Radioactive Decay
Radioactive decay affects the environment in various ways, both positively and negatively.
-
Radioactive isotopes help scientists study environmental processes, such as soil erosion and water movement.
-
Nuclear fallout from atomic bomb tests and accidents, like Chernobyl and Fukushima, has long-lasting environmental consequences.
-
Radioactive contamination can affect ecosystems, leading to mutations and reduced biodiversity.
-
Phytoremediation, using plants to absorb radioactive contaminants, is a promising method for cleaning up contaminated sites.
-
Natural background radiation from cosmic rays and radioactive elements in the Earth's crust contributes to the overall radiation exposure.
Fun and Surprising Facts About Radioactive Decay
Radioactive decay can be surprising and even a bit fun when you learn about its quirks and oddities.
-
Bananas contain potassium-40, a naturally occurring radioactive isotope. Eating a banana exposes you to a tiny amount of radiation.
-
Brazil nuts are one of the most radioactive foods due to their high radium content.
-
The Oklo natural nuclear reactor in Gabon, Africa, is a 2-billion-year-old natural reactor where self-sustaining nuclear reactions occurred.
-
Tritium, a radioactive form of hydrogen, is used in glow-in-the-dark watches and exit signs.
-
The "Curie" is a unit of radioactivity named after Marie Curie. One Curie equals 37 billion disintegrations per second.
-
The "banana equivalent dose" is a humorous way to explain radiation exposure. One BED equals the radiation dose from eating one banana.
-
Glow-in-the-dark paint used in watches and clocks in the early 20th century contained radium, leading to health issues for workers who painted the dials.
The Fascinating World of Radioactive Decay
Radioactive decay isn't just a scientific concept; it's a window into the mysteries of our universe. From powering nuclear reactors to helping us understand the age of ancient artifacts, this process plays a crucial role in many aspects of our lives. Elements like uranium and radon might sound intimidating, but they offer insights into both the dangers and benefits of radioactivity. Understanding half-lives, isotopes, and decay chains can demystify the science behind it all.
Whether it's medical applications, energy production, or environmental studies, radioactive decay has far-reaching implications. It's a reminder of the delicate balance between harnessing nature's power and respecting its potential hazards. So next time you hear about radioactivity, remember it's not just about danger; it's also about discovery, innovation, and the incredible ways we can use science to improve our world.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
