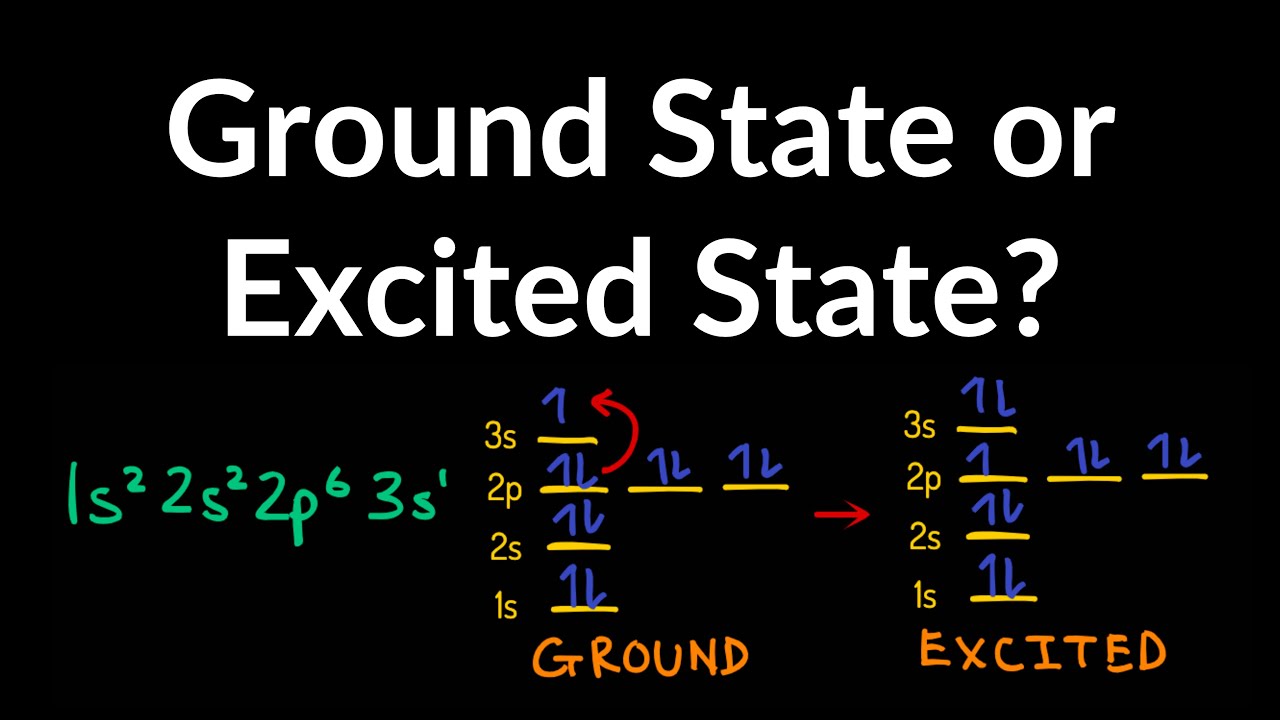
What are excited states, and why are they important?
Excited states occur when electrons in an atom or molecule absorb energy and jump to higher energy levels. This phenomenon is crucial because it plays a key role in various scientific fields, including chemistry, physics, and even biology. For instance, the colors we see in fireworks or neon signs are due to electrons returning from excited states to their ground states, releasing energy as light. Understanding excited states helps scientists develop new materials, improve solar cells, and even create medical imaging techniques. Without this knowledge, many modern technologies would not exist.
What Are Excited States?
When atoms or molecules absorb energy, they move to a higher energy level, known as an excited state. This phenomenon plays a crucial role in various scientific fields, from chemistry to physics. Let's dive into some fascinating facts about excited states.
-
Energy Absorption: Atoms or molecules enter an excited state when they absorb energy, typically from light or heat.
-
Short-Lived: Excited states are usually short-lived, lasting only fractions of a second before returning to a lower energy state.
-
Photon Emission: When returning to a lower energy state, atoms or molecules often emit photons, producing light.
-
Quantum Mechanics: The concept of excited states is deeply rooted in quantum mechanics, which describes the behavior of particles at atomic and subatomic levels.
-
Fluorescence: Many substances exhibit fluorescence, where they absorb light at one wavelength and emit it at another, due to excited states.
-
Phosphorescence: Unlike fluorescence, phosphorescence involves a delayed emission of light, lasting from milliseconds to hours.
-
Spectroscopy: Scientists use spectroscopy to study excited states by analyzing the light emitted or absorbed by substances.
-
Chemical Reactions: Excited states can drive chemical reactions, providing the necessary energy to break and form bonds.
-
Photosynthesis: In plants, excited states of chlorophyll molecules play a vital role in converting sunlight into chemical energy.
-
Lasers: Lasers rely on excited states to produce coherent light through a process called stimulated emission.
Types of Excited States
Excited states can vary depending on the type of energy absorbed and the specific atom or molecule involved. Here are some different types of excited states.
-
Electronic Excited States: Involve the promotion of an electron to a higher energy orbital.
-
Vibrational Excited States: Occur when molecules absorb energy and vibrate at higher frequencies.
-
Rotational Excited States: Happen when molecules rotate faster after absorbing energy.
-
Triplet States: A type of electronic excited state where electrons have parallel spins, often leading to longer-lived states.
-
Singlet States: Another type of electronic excited state where electrons have opposite spins, usually shorter-lived than triplet states.
-
Rydberg States: Highly excited electronic states where an electron is in a very high orbital, far from the nucleus.
-
Excitons: Quasi-particles formed in semiconductors when an electron and a hole pair up in an excited state.
-
Polaritons: Hybrid states resulting from the coupling of photons with excitons in certain materials.
Applications of Excited States
Excited states have numerous practical applications in technology, medicine, and research. Here are some examples.
-
Medical Imaging: Techniques like PET scans use excited states to visualize internal body structures.
-
Solar Cells: Excited states in photovoltaic materials help convert sunlight into electricity.
-
OLEDs: Organic light-emitting diodes rely on excited states to produce bright, energy-efficient displays.
-
Quantum Computing: Excited states of qubits are essential for performing quantum computations.
-
Photodynamic Therapy: Uses light to activate drugs in excited states, targeting cancer cells.
-
Molecular Probes: Fluorescent probes in biological research rely on excited states to tag and visualize molecules.
-
Environmental Monitoring: Sensors detecting pollutants often use excited states to identify specific chemicals.
-
Forensic Science: Techniques like luminescence spectroscopy use excited states to analyze crime scene evidence.
Interesting Phenomena Involving Excited States
Excited states lead to some intriguing and sometimes unexpected phenomena. Here are a few worth noting.
-
Auroras: The beautiful northern and southern lights result from excited states of atmospheric gases.
-
Bioluminescence: Some organisms, like fireflies, produce light through biochemical reactions involving excited states.
-
Chemiluminescence: Chemical reactions that produce light, such as glow sticks, involve excited states.
-
Afterglow: Certain materials continue to emit light after the excitation source is removed, due to persistent excited states.
-
Quantum Dots: Nanoscale semiconductor particles exhibit unique optical properties due to their excited states.
-
Stokes Shift: The difference in wavelength between absorbed and emitted light in fluorescence, caused by excited states.
-
Non-Radiative Decay: Excited states can lose energy without emitting light, transferring it as heat instead.
-
Energy Transfer: Excited states can transfer energy to nearby molecules, a process crucial in photosynthesis and other biological systems.
-
Two-Photon Absorption: Some materials can absorb two photons simultaneously, reaching an excited state with higher energy.
-
Upconversion: A process where lower-energy photons combine to excite a molecule to a higher energy state, emitting higher-energy light.
Final Thoughts on Excited States
Excited states are fascinating. They play a crucial role in many scientific fields, from quantum mechanics to chemistry. When atoms or molecules absorb energy, they jump to a higher energy level. This process is essential for photosynthesis, fluorescence, and even lasers. Understanding these states helps scientists develop new technologies and materials.
Remember, excited states are temporary. Eventually, atoms return to their ground state, releasing energy. This energy release can be in the form of light, heat, or other types of radiation.
So, next time you see a glowing neon sign or enjoy the vibrant colors of a fireworks display, you'll know the science behind the spectacle. Excited states might seem complex, but they make our world a lot more interesting. Keep exploring and stay curious!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
