Isotopes are fascinating variations of elements that share the same number of protons but differ in neutron count. This difference gives each isotope unique properties, making them crucial in various fields like medicine, archaeology, and energy production. Did you know that carbon-14 helps date ancient artifacts, while uranium-235 powers nuclear reactors? These tiny differences in atomic structure have massive impacts on our daily lives. From diagnosing illnesses with radioactive tracers to understanding climate change through ice core samples, isotopes play a pivotal role. Ready to dive into 33 intriguing facts about these atomic wonders? Let's get started!
What Are Isotopes?
Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons. This means they have the same atomic number but different mass numbers. Let's dive into some fascinating facts about isotopes.
-
Isotopes of an element share the same chemical properties but differ in physical properties due to their mass differences.
-
The term "isotope" comes from the Greek words "isos" (equal) and "topos" (place), meaning they occupy the same place on the periodic table.
-
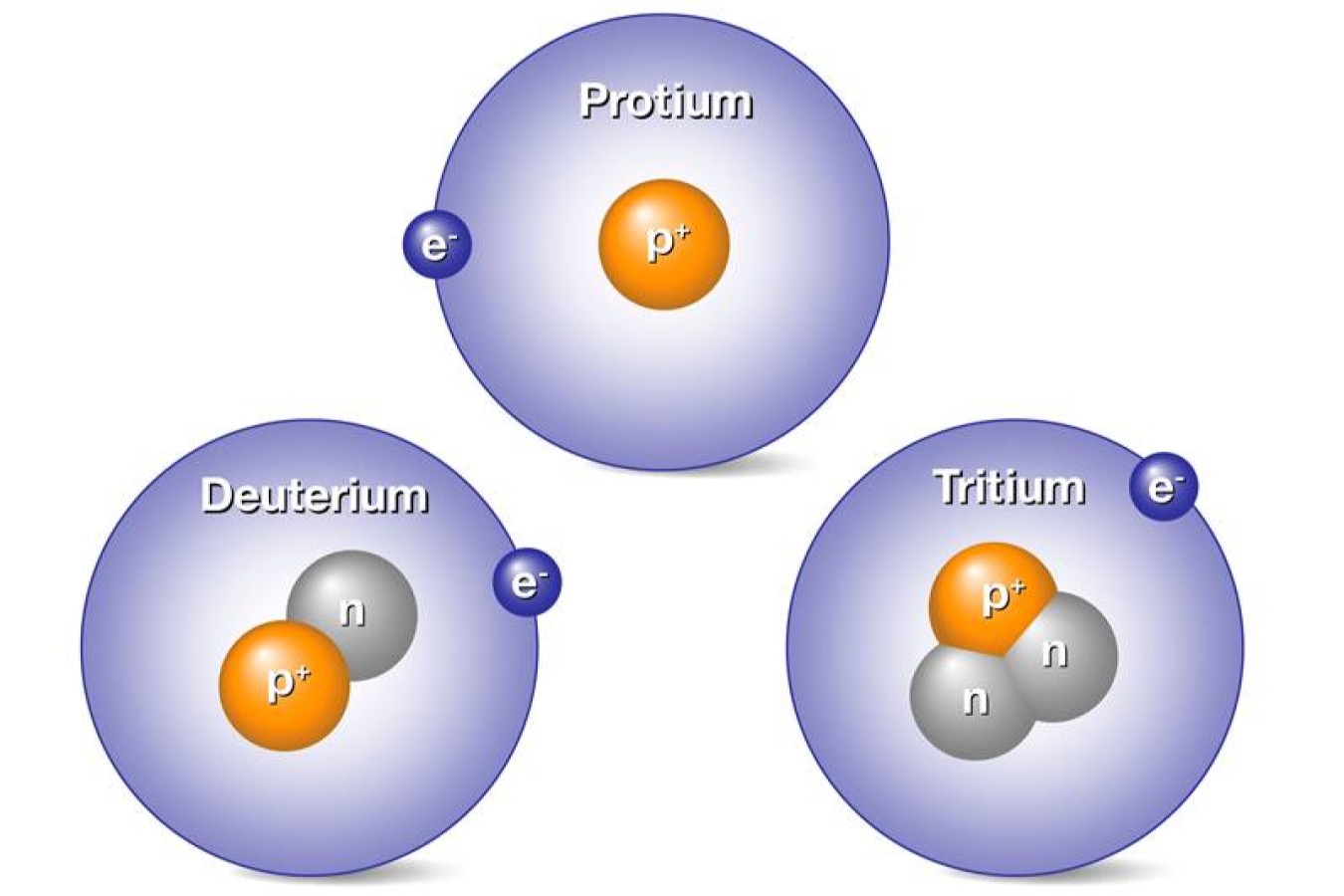
Hydrogen has three common isotopes: protium, deuterium, and tritium. Protium has no neutrons, deuterium has one, and tritium has two.
Natural and Artificial Isotopes
Isotopes can be found naturally or created artificially. Natural isotopes occur in nature, while artificial ones are produced in laboratories or nuclear reactors.
-
Carbon-12 and Carbon-14 are isotopes of carbon. Carbon-14 is used in radiocarbon dating to determine the age of archaeological finds.
-
Uranium-235 and Uranium-238 are isotopes of uranium. Uranium-235 is used as fuel in nuclear reactors and atomic bombs.
-
Technetium-99m is an artificial isotope used in medical imaging to diagnose various conditions.
Radioactive Isotopes
Some isotopes are radioactive, meaning they decay over time and emit radiation. These are known as radioisotopes.
-
Radon-222 is a radioactive isotope that is a health hazard due to its radioactive decay, which can lead to lung cancer.
-
Cobalt-60 is a radioisotope used in cancer treatment and sterilization of medical equipment.
-
Iodine-131 is used in the treatment of thyroid disorders, including thyroid cancer.
Stable Isotopes
Not all isotopes are radioactive. Stable isotopes do not undergo radioactive decay and remain constant over time.
-
Oxygen has three stable isotopes: Oxygen-16, Oxygen-17, and Oxygen-18. These are used in climate studies to understand past temperatures.
-
Nitrogen-14 and Nitrogen-15 are stable isotopes of nitrogen. Nitrogen-15 is used in agricultural research to study nitrogen cycles.
-
Magnesium has three stable isotopes: Magnesium-24, Magnesium-25, and Magnesium-26.
Isotopes in Everyday Life
Isotopes play a significant role in various fields, including medicine, archaeology, and environmental science.
-
Deuterium, an isotope of hydrogen, is used in heavy water reactors as a neutron moderator.
-
Carbon-13 is used in metabolic research to study biochemical pathways.
-
Sulfur isotopes are used in environmental studies to track pollution sources.
Isotopes in Space
Isotopes are not just limited to Earth; they are found throughout the universe and provide valuable information about cosmic events.
-
Helium-3 is a rare isotope found on the Moon and is considered a potential fuel for future nuclear fusion reactors.
-
Iron-60 is an isotope found in meteorites, providing clues about the formation of our solar system.
-
Aluminum-26 is used to study the age and formation of meteorites and planetary bodies.
Isotopes in Research
Scientists use isotopes in various research fields to gain insights and make groundbreaking discoveries.
-
Isotopic labeling is a technique where isotopes are used to track the passage of an element through a system.
-
Isotopes are used in paleoclimatology to study past climate changes by analyzing ice cores and sediment layers.
-
In biochemistry, isotopes help in understanding enzyme mechanisms and metabolic pathways.
Fun Facts About Isotopes
Isotopes have some quirky and interesting aspects that make them even more fascinating.
-
The isotope ratio of oxygen in water can tell scientists about the water's origin and history.
-
Some isotopes are so rare that only a few atoms have ever been observed.
-
Isotopes can be used to authenticate artworks by analyzing the isotopic composition of the materials used.
Isotopes in Industry
Industries utilize isotopes for various applications, from quality control to energy production.
-
Americium-241 is used in smoke detectors to ionize air and detect smoke particles.
-
Iridium-192 is used in industrial radiography to inspect metal parts and welds for defects.
-
Plutonium-238 is used as a heat source in radioisotope thermoelectric generators, which power spacecraft.
Isotopes in Agriculture
Agriculture benefits from isotopes in improving crop yields and understanding soil health.
-
Nitrogen-15 is used to study nitrogen fixation in plants, helping improve fertilizer use.
-
Carbon-13 is used to study photosynthesis and plant respiration.
-
Isotopes help in tracing the movement of water and nutrients in soil.
Isotopes in Forensics
Forensic scientists use isotopes to solve crimes and identify unknown individuals.
-
Strontium isotopes in human teeth and bones can reveal geographical origins and migration patterns.
-
Isotopic analysis of hair can provide information about a person's diet and lifestyle.
-
Lead isotopes can help trace the source of lead poisoning in criminal investigations.
The Fascinating World of Isotopes
Isotopes are more than just scientific jargon. They play crucial roles in medicine, archaeology, environmental science, and even energy production. From treating cancer with radioactive isotopes to dating ancient artifacts, their applications are vast and varied. Understanding isotopes helps us grasp the complexities of the natural world and the universe.
Next time you hear about carbon-14 dating or medical imaging, you'll know isotopes are behind these incredible technologies. They might seem like a small part of the atomic world, but their impact is enormous. So, whether you're a student, a science enthusiast, or just curious, keep exploring the wonders of isotopes. They truly are a key to unlocking many of the mysteries around us.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
