Statistical mechanics might sound like a complex topic, but it's all about understanding how large groups of particles behave. Imagine trying to predict the weather by looking at every single air molecule—impossible, right? Instead, scientists use statistical mechanics to make sense of the chaos. This field combines physics and probability to explain how things like temperature and pressure come from the motion of countless tiny particles. Statistical mechanics helps us understand everything from why ice melts to how engines work. Ready to dive into some cool facts about this fascinating subject? Let's break it down into bite-sized pieces!
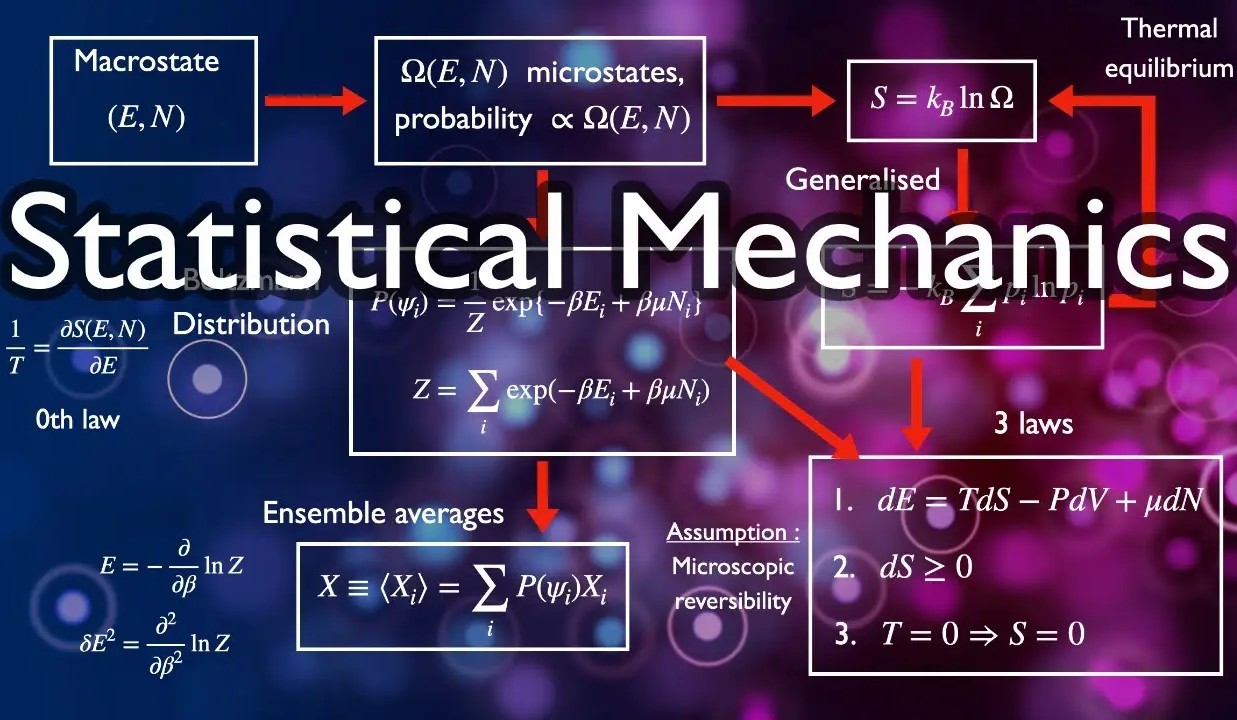
What is Statistical Mechanics?
Statistical mechanics bridges the gap between microscopic and macroscopic worlds. It uses statistics to explain how particles behave in a system. This field is crucial for understanding thermodynamics and quantum mechanics.
- Statistical mechanics combines principles from physics and mathematics to study large systems.
- It explains how microscopic particles like atoms and molecules result in macroscopic properties like temperature and pressure.
- Ludwig Boltzmann and James Clerk Maxwell are pioneers in this field.
- The Boltzmann constant is a fundamental constant in statistical mechanics.
Key Concepts in Statistical Mechanics
Understanding the core ideas helps grasp the subject better. These concepts are foundational to the field.
- Microstates refer to specific configurations of particles in a system.
- Macrostates are the observable properties of a system, like temperature and volume.
- Entropy measures the disorder or randomness in a system.
- Partition function is a crucial mathematical tool used to sum up all possible states of a system.
Applications of Statistical Mechanics
This field isn't just theoretical; it has practical applications in various domains.
- Thermodynamics relies heavily on statistical mechanics for explaining heat and work.
- Quantum mechanics uses statistical principles to describe particle behavior at atomic scales.
- Material science benefits from understanding the properties of solids, liquids, and gases.
- Biophysics applies these principles to study biological systems like proteins and DNA.
Important Theorems and Laws
Several theorems and laws form the backbone of statistical mechanics. These are essential for solving complex problems.
- The Ergodic hypothesis states that over time, a system will explore all accessible microstates.
- Boltzmann's H-theorem explains the increase of entropy over time.
- Gibbs' phase rule helps in understanding phase transitions in materials.
- Fermi-Dirac statistics describe particles that follow the Pauli exclusion principle, like electrons.
- Bose-Einstein statistics apply to particles that can occupy the same state, like photons.
Real-World Examples
Statistical mechanics isn't confined to textbooks. It explains many phenomena we encounter daily.
- Weather patterns can be analyzed using statistical mechanics to predict changes.
- Chemical reactions are better understood by studying the energy states of molecules.
- Magnetic properties of materials are explained through spin states and interactions.
- Superconductivity is a phenomenon where materials conduct electricity without resistance, explained by Bose-Einstein condensation.
Challenges and Future Directions
Despite its success, statistical mechanics faces challenges and has room for growth.
- Non-equilibrium systems are harder to analyze compared to equilibrium systems.
- Complex systems like biological organisms present unique challenges.
- Quantum computing could revolutionize the way statistical mechanics problems are solved.
- Machine learning is being integrated to predict and analyze complex systems more efficiently.
Fun Facts
Let's end with some interesting tidbits about statistical mechanics.
- Boltzmann's tombstone features his famous entropy formula, ( S = k log W ).
- Einstein used statistical mechanics to explain the photoelectric effect, which won him the Nobel Prize.
The Final Word on Statistical Mechanics
Statistical mechanics bridges microscopic particle behavior with macroscopic phenomena. It explains how gas molecules move, why ice melts, and how stars shine. This field combines physics, mathematics, and probability to predict system behaviors. From the laws of thermodynamics to the concept of entropy, statistical mechanics offers a deep understanding of nature's rules.
Understanding these principles can lead to advancements in technology, energy efficiency, and even climate science. It's not just for scientists; anyone curious about how the world works can appreciate its insights. Whether you're a student, a researcher, or just someone who loves learning, statistical mechanics provides a fascinating glimpse into the hidden workings of the universe.
So next time you see steam rising from a cup of coffee or marvel at the stars, remember there's a whole world of particles and probabilities making it all happen.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
