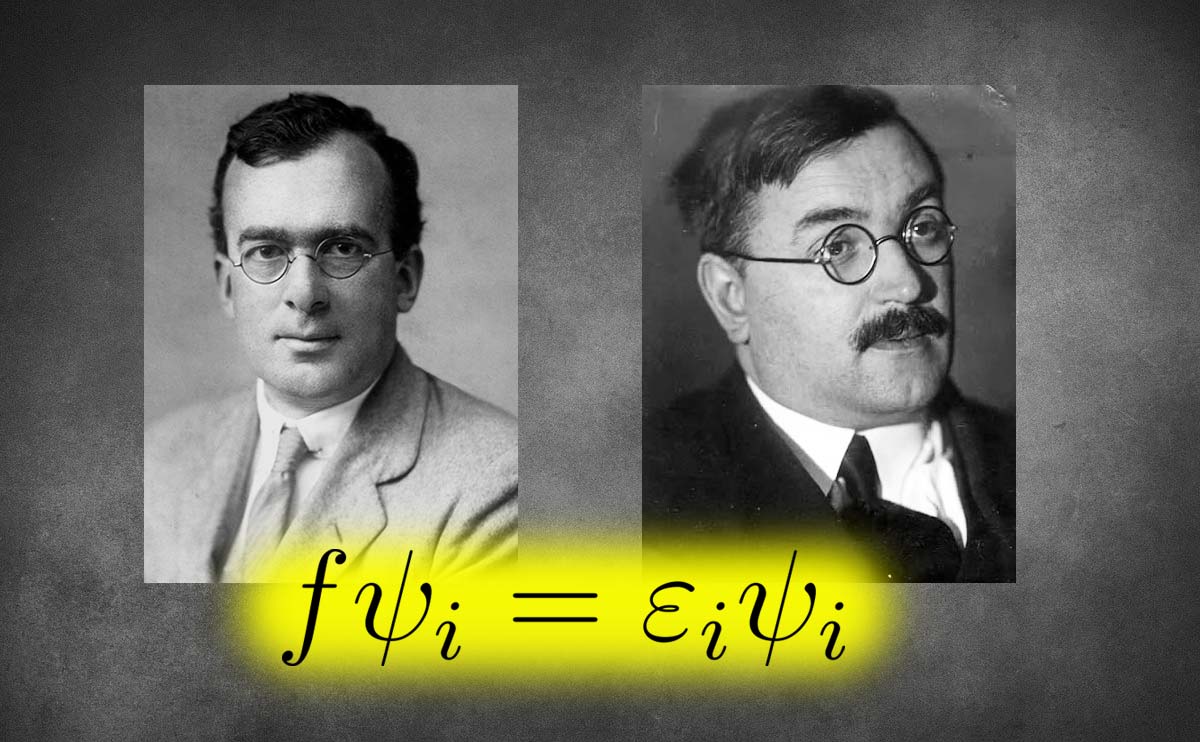
What is the Hartree-Fock method? The Hartree-Fock method is a computational approach used in quantum chemistry to approximate the wave functions and energy of a quantum many-body system. It simplifies the complex interactions between electrons by assuming each electron moves independently in an average field created by all other electrons. This method helps predict molecular structures, chemical reactions, and properties of atoms and molecules. By solving the Hartree-Fock equations, scientists can gain insights into the electronic structure of molecules, making it a crucial tool in theoretical chemistry. Understanding this method can help demystify the behavior of electrons in various chemical environments.
What is the Hartree-Fock Method?
The Hartree-Fock method is a cornerstone in quantum chemistry. It helps scientists understand the behavior of electrons in atoms and molecules. Let's dive into some fascinating facts about this method.
-
The Hartree-Fock method is named after physicists Douglas Hartree and Vladimir Fock, who developed it in the 1920s and 1930s.
-
This method approximates the wave function of a multi-electron system using a single Slater determinant, simplifying complex calculations.
-
It is a mean-field theory, meaning each electron feels an average effect of all other electrons rather than individual interactions.
-
The Hartree-Fock method is the starting point for more advanced quantum chemistry methods like Configuration Interaction (CI) and Coupled Cluster (CC).
How Does the Hartree-Fock Method Work?
Understanding the mechanics of the Hartree-Fock method can be quite intriguing. Here are some key points about its working principles.
-
The method solves the Schrödinger equation for a multi-electron system by transforming it into a set of simpler equations.
-
It uses the concept of self-consistency, where the solution is iteratively refined until it converges to a stable state.
-
The Fock operator, a crucial component, includes both kinetic energy and potential energy terms.
-
Electron correlation is not fully accounted for in the Hartree-Fock method, which can lead to inaccuracies for certain systems.
Applications of the Hartree-Fock Method
The Hartree-Fock method has a wide range of applications in chemistry and physics. Here are some areas where it plays a vital role.
-
It is used to calculate the electronic structure of atoms, molecules, and solids.
-
The method helps in predicting molecular properties like bond lengths, angles, and dipole moments.
-
It is essential for understanding reaction mechanisms and energy barriers in chemical reactions.
-
The Hartree-Fock method is also used in the study of excited states and electronic transitions.
Advantages of the Hartree-Fock Method
Despite its limitations, the Hartree-Fock method offers several advantages that make it a valuable tool in quantum chemistry.
-
It provides a good starting point for more accurate post-Hartree-Fock methods.
-
The method is relatively simple and computationally less demanding compared to more advanced techniques.
-
It offers a clear physical interpretation of the electronic structure of systems.
-
The Hartree-Fock method can be applied to a wide range of systems, from small molecules to large biological macromolecules.
Limitations of the Hartree-Fock Method
No method is perfect, and the Hartree-Fock method has its own set of limitations. Here are some of the challenges associated with it.
-
It neglects electron correlation, which can lead to significant errors in certain cases.
-
The method assumes that electrons move independently, which is not always a valid approximation.
-
It can struggle with systems that have near-degenerate electronic states.
-
The Hartree-Fock method may not accurately predict properties of strongly correlated systems like transition metal complexes.
Improvements and Extensions of the Hartree-Fock Method
Over the years, several improvements and extensions have been developed to overcome the limitations of the Hartree-Fock method.
-
Post-Hartree-Fock methods like Møller-Plesset perturbation theory (MP2) and Coupled Cluster (CC) include electron correlation effects.
-
Density Functional Theory (DFT) offers an alternative approach that can handle electron correlation more effectively.
-
Multi-configurational methods like Complete Active Space Self-Consistent Field (CASSCF) are used for systems with near-degenerate states.
-
Hybrid methods combine Hartree-Fock with other techniques to achieve better accuracy and efficiency.
-
Advances in computational power and algorithms continue to enhance the applicability and accuracy of the Hartree-Fock method and its extensions.
Final Thoughts on Hartree-Fock Method
The Hartree-Fock method stands as a cornerstone in quantum chemistry. It simplifies the complex interactions of electrons in atoms and molecules, making calculations more manageable. By approximating the wave functions of a multi-electron system, it provides a foundation for more advanced methods like Density Functional Theory and Coupled Cluster Theory.
Understanding its limitations, such as the neglect of electron correlation, is crucial. Despite this, its efficiency and relatively straightforward implementation make it invaluable for many chemical and physical applications. Whether you're a student, researcher, or enthusiast, grasping the basics of Hartree-Fock can deepen your appreciation of the microscopic world.
In essence, the Hartree-Fock method is a powerful tool that bridges the gap between theoretical concepts and practical applications in quantum chemistry. Its impact on the field is undeniable, continuing to influence research and development.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
