For centuries, humans have been intrigued by the laws that govern the universe. One such law is Coulomb’s Law of Electric Interaction, which provides a mathematical description of the force between charged particles. Proposed by French physicist Charles-Augustin de Coulomb in the late 18th century, this fundamental law has revolutionized our understanding of electricity and magnetism.
In this article, we will delve into the enigmatic world of Coulomb’s Law and explore 17 fascinating facts about this profound concept. From its historical significance to its practical applications in various fields, get ready to unravel the mysteries surrounding this iconic law. So, fasten your seatbelts and get ready for an electrifying journey into the intriguing world of Coulomb’s Law!
Key Takeaways:
- Coulomb’s Law explains how electric charges interact, with force increasing as charges get bigger and decreasing as distance grows. It’s like a secret code for understanding electricity!
- Just like magnets, electric charges attract or repel based on their type. Coulomb’s Law helps us understand this and even led to cool inventions like capacitors and electric generators.
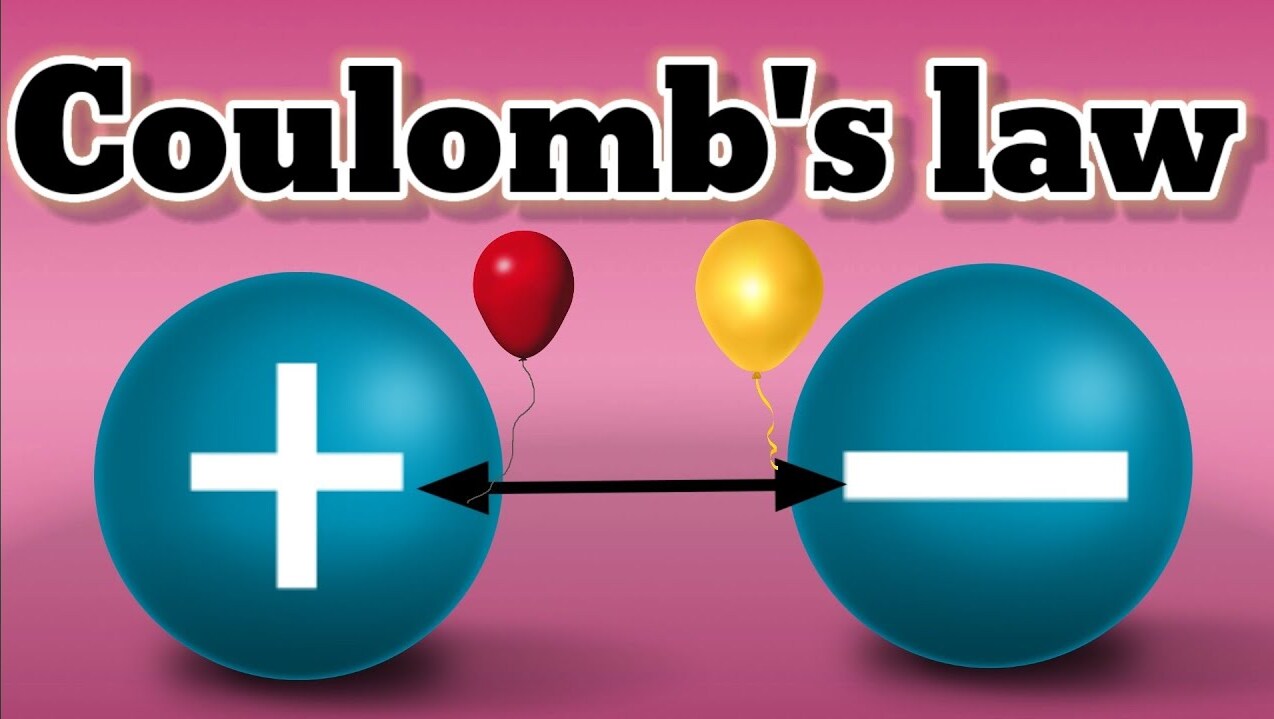
Coulomb’s Law defines the interaction between electric charges.
Coulomb’s Law, named after Charles-Augustin de Coulomb, is a fundamental principle in physics that describes the electrical interaction between charged particles.
The law states that the force between two charged objects is directly proportional to the product of their charges.
This means that the force increases as the charges of the objects increase.
The force between charged objects decreases as the distance between them increases.
This inverse relationship between the force and distance is a crucial aspect of Coulomb’s Law.
Coulomb’s Law can be expressed mathematically as F = k * (q1 * q2) / r^2.
Here, F represents the force between the two charges, q1 and q2 represent the magnitudes of the charges, r is the distance between them, and k is the proportionality constant.
The proportionality constant, k, is known as Coulomb’s constant and is approximately equal to 8.99 × 10^9 N·m^2/C^2.
This constant allows us to quantify the force between charged objects.
Coulomb’s Law applies to both positive and negative charges.
Whether the charges are of the same sign (repulsive) or opposite sign (attractive), the law applies to both scenarios.
The direction of the force between charged objects depends on their charges.
Like charges repel each other, while opposite charges attract.
Coulomb’s Law follows the principle of superposition.
This means that the total force between multiple charged objects is the vector sum of the individual forces acting on each pair of charges.
Coulomb’s Law is essential for understanding the behavior of static electricity.
From the attraction of clothes after they are removed from a dryer to the phenomenon of lightning, Coulomb’s Law plays a significant role in explaining these everyday occurrences.
Coulomb’s Law is a fundamental principle in electrostatics and electromagnetism.
It provides a basis for many other concepts and laws in these fields of physics.
The similarities between Coulomb’s Law and Newton’s Law of Universal Gravitation are striking.
Both laws follow an inverse-square relationship and involve a proportionality constant.
Coulomb’s Law led to the development of the concept of an electric field.
By considering the force per unit charge, physicists extended the idea of electric interaction to the notion of an electric field surrounding charged objects.
Coulomb’s Law remains valid over a wide range of distances.
Whether the charges are close together or far apart, the law accurately predicts the force between them.
The discovery of Coulomb’s Law paved the way for advancements in the field of electrostatics.
It laid the foundation for understanding the behavior of charges and the development of technologies such as capacitors and electric generators.
Coulomb’s Law applies to point charges.
While real-world objects may have distributed charges, in many cases, approximating them as point charges allows us to apply Coulomb’s Law effectively.
Coulomb’s Law helps explain the behavior of atomic and molecular systems.
From the stability of atoms to the interactions between molecules, the law provides insights into the forces at play.
The principles underlying Coulomb’s Law extend to more complex electrical systems.
From circuits and electronics to the behavior of charged particles in accelerators, the foundational concepts of Coulomb’s Law find applications in various branches of physics.
Conclusion
In conclusion, Coulomb’s Law of Electric Interaction is a fundamental principle in physics that describes the force between charged particles. It provides valuable insights into the behavior of electric charges and is widely applicable in various fields, including electromagnetism, engineering, and even biology. Its mathematical formulation allows scientists to calculate and predict the strength and direction of the electric force, enabling the development of numerous technological advancements.
Understanding the enigmatic facts about Coulomb’s Law not only deepens our knowledge of electricity and magnetism but also paves the way for innovative discoveries and applications. From the inverse square relationship to the role of medium in electric interactions, these facts highlight the intricacies and significance of Coulomb’s Law in the physical world.
By delving into the mysteries of Coulomb’s Law, we gain a deeper appreciation for the elegance and complexity of the laws that govern the universe.
FAQs
Q: What is Coulomb’s Law?
A: Coulomb’s Law is a fundamental principle in physics that describes the force between charged particles. It states that the force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them.
Q: What is the mathematical form of Coulomb’s Law?
A: Coulomb’s Law is mathematically represented as F = k * (q1 * q2) / r^2, where F is the force between two charges, q1 and q2 are the magnitudes of the charges, r is the distance between them, and k is the electrostatic constant.
Q: What is the significance of Coulomb’s Law?
A: Coulomb’s Law forms the foundation for understanding electric interactions, helping scientists calculate and predict the forces between charged particles. It has numerous practical applications in fields such as electrical engineering, magnetism, and electronics.
Q: What is the inverse square relationship in Coulomb’s Law?
A: The inverse square relationship states that the force between two charges decreases with the square of the distance between them. In simpler terms, doubling the distance between the charges will result in the force being four times weaker.
Q: What is the role of the medium in electric interactions?
A: The medium between charged particles can have an impact on electric interactions. The presence of a medium can affect the effective charge, altering the magnitude and direction of the electric force.
Q: Can Coulomb’s Law be applied to situations beyond point charges?
A: Coulomb’s Law is valid for point charges, but it can also be used to approximate the behavior of non-point charges as long as their dimensions are much smaller than the distance between them.
Coulomb's Law unveils secrets of electric interaction, but there's more to explore! Dive into the captivating world of physics/8-captivating-facts-about-electric-charge/">electric charge and uncover its mysteries. Journey through the realm of physics, where fascinating phenomena await. Ready for more electrifying knowledge? Discover the intriguing facts about Coulomb's Law of electrostatics and deepen your understanding of this fundamental principle.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
