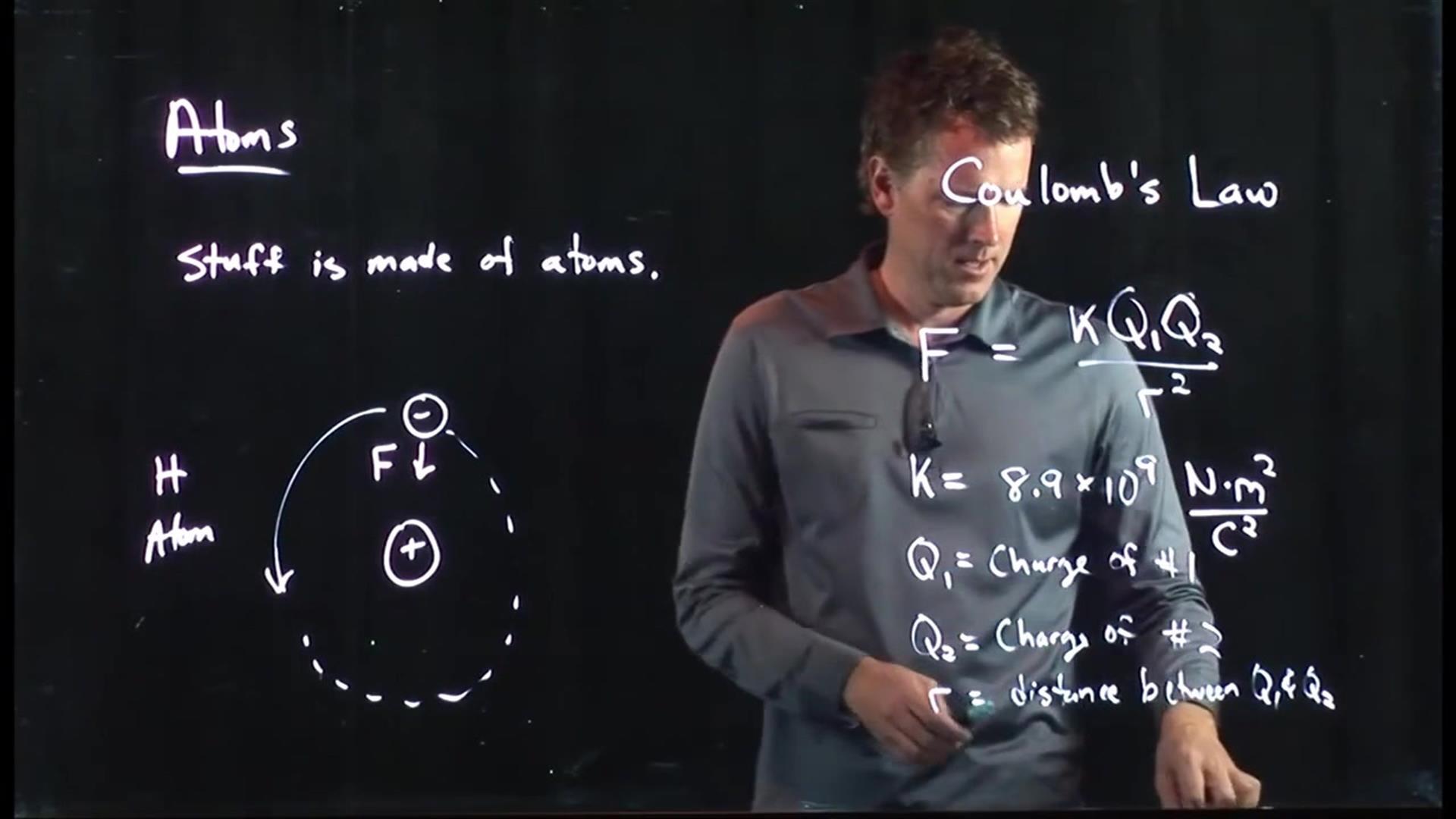
When it comes to understanding the fundamental forces that govern our universe, Coulomb’s Law holds a significant place in the realm of physics. Named after the French physicist Charles-Augustin de Coulomb, this law describes the interactions between electric charges and the force they exert on each other. Its discovery revolutionized the field of electromagnetism and laid the foundation for many key scientific breakthroughs.
In this article, we will delve into 13 astounding facts about Coulomb’s Law that will not only deepen your understanding of this principle but also showcase its profound impact on modern science and technology. From the historical origins of the law to its practical applications in everyday life, prepare to be amazed by the intricacies of Coulomb’s Law and its far-reaching implications.
Key Takeaways:
- Coulomb’s law, discovered by physicist Charles-Augustin de Coulomb, explains how electric charges interact. It’s like a secret formula for understanding the behavior of electricity!
- Coulomb’s law is not just a historical discovery; it’s a crucial tool used in fields like electronics, particle physics, and engineering. It’s like the superhero of the electromagnetic universe!
The discovery of Coulomb’s law revolutionized the field of electrostatics.
Coulomb’s law, formulated by French physicist Charles-Augustin de Coulomb in the late 18th century, laid the foundation for understanding the behavior of electric charges.
Coulomb’s law is based on the inverse square relationship between electric charges.
According to Coulomb’s law, the force between two charged objects is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
Coulomb’s law applies to both point charges and charged objects.
Whether the charges are concentrated at a single point or distributed over an object, Coulomb’s law can be used to calculate the electric force between them.
The SI unit of electric charge, the coulomb (C), is named after Charles-Augustin de Coulomb.
This unit is used to measure the amount of charge possessed by an object or the quantity of charge involved in an electric interaction.
Coulomb’s law forms the foundation of Gauss’s law in electrostatics.
Gauss’s law provides a mathematical relationship between the distribution of electric charges and the resulting electric field.
Coulomb’s law is applicable not only to electric charges but also to static magnetic poles.
Magnetic poles, similar to electric charges, experience forces that obey the same inverse square relationship.
Coulomb’s law is symmetric, meaning the magnitude of the force between two charges is the same regardless of their order.
For example, the force between charge A and charge B is equal in magnitude to the force between charge B and charge A.
Coulomb’s law can be used to calculate the electric field around a charged object.
By using Coulomb’s law to determine the electric force at various points around a charge, the electric field at those points can be determined.
Coulomb’s law breaks down at extremely small distances.
At the subatomic level, where quantum effects dominate, Coulomb’s law is superseded by quantum electrodynamics (QED), which describes the behavior of electromagnetic forces.
Coulomb’s law finds applications in various fields, including electronics, particle physics, and astronomy.
From designing electronic circuits to studying the behavior of subatomic particles and celestial bodies, Coulomb’s law is fundamental in understanding and analyzing electric and electromagnetic phenomena.
Coulomb’s law is a fundamental principle in the study of electric fields and forces.
It forms the basis for further exploration into topics such as electrical potential, capacitance, and the behavior of conductors and insulators.
Coulomb’s law is an essential tool in engineering applications like designing electrical systems and predicting the behavior of charged particles in accelerators.
Engineers and physicists rely on the principles of Coulomb’s law to ensure the proper functioning of electrical devices and to model the trajectories of charged particles in particle accelerators.
Coulomb’s law provides a quantitative understanding of one of the fundamental forces of nature.
By quantifying the relationship between electric charges, Coulomb’s law bridges the gap between theoretical concepts and practical applications, helping us unravel the mysteries of the electromagnetic universe.
In conclusion, these 13 astounding facts about Coulomb’s Law showcase the profound impact it has had on our understanding of electromagnetic interactions. From its revolutionary discovery to its wide range of applications, Coulomb’s law continues to be a cornerstone of physics and engineering.
Conclusion
Coulomb’s Law is a fundamental concept in physics that governs the interaction between electric charges. Its discovery by Charles-Augustin de Coulomb in the 18th century laid the groundwork for understanding the nature of electric forces. We have explored 13 astounding facts about Coulomb’s Law, shedding light on its significance and implications in the world of physics. From its mathematical formulation to its application in various fields, Coulomb’s Law remains a cornerstone of electromagnetism. Understanding this law has enabled scientists to develop technologies like electric motors, generators, and even particle accelerators. The study of Coulomb’s Law continues to deepen our understanding of the complex forces that shape the world around us. As we delve deeper into the realm of electricity and magnetism, we can appreciate the profound impact of Coulomb’s Law in unlocking the secrets of the universe.
FAQs
1. What is Coulomb’s Law?
Coulomb’s Law states that the force of interaction between two charged objects is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
2. What is the mathematical expression of Coulomb’s Law?
The mathematical expression of Coulomb’s Law is F = k * q1 * q2 / r^2, where F is the force of interaction, q1 and q2 are the charges of the objects, r is the distance between them, and k is the electrostatic constant.
3. What is the unit of charge used in Coulomb’s Law?
The unit of charge used in Coulomb’s Law is the Coulomb (C).
4. Can Coulomb’s Law be applied to both positive and negative charges?
Yes, Coulomb’s Law can be applied to both positive and negative charges. The force between like charges is repulsive, while the force between unlike charges is attractive.
5. Is Coulomb’s Law only applicable to point charges?
No, Coulomb’s Law is not limited to point charges. It can be extended to charged objects of any shape or size, as long as the charges are distributed uniformly.
6. Is Coulomb’s Law applicable to non-static charges in motion?
No, Coulomb’s Law is not directly applicable to charges in motion. It describes the static electrostatic forces between charges at rest.
7. How does Coulomb’s Law relate to electric fields?
Coulomb’s Law is closely related to electric fields. The electric field created by a charged object is a measure of the force experienced by another charged object placed in that field.
8. Does Coulomb’s Law apply to all scales, from atomic to astronomical?
Yes, Coulomb’s Law applies to all scales, from atomic to astronomical. It governs the interactions between charged particles at all levels of magnitude.
9. Can Coulomb’s Law be applied to magnetic forces?
No, Coulomb’s Law is specific to electric forces. Magnetic forces are governed by a different set of laws known as Ampere’s Law and Biot-Savart Law.
10. How does Coulomb’s Law contribute to the development of technology?
Coulomb’s Law has contributed to the development of technology in various ways. It forms the basis of electric circuits, electrical motors, generators, and many other electrical devices we use in our daily lives.
11. Is Coulomb’s Law valid only in a vacuum?
No, Coulomb’s Law is valid in any medium, including gases, liquids, and solids. However, the presence of a medium may modify the electrostatic interactions to some extent.
12. Are there any practical applications of Coulomb’s Law?
Yes, Coulomb’s Law has practical applications in several fields. It is used in designing electrical systems, analyzing atomic and molecular structures, studying particle physics, and even in biochemistry and medicine.
13. Can Coulomb’s Law be used to explain gravity?
No, Coulomb’s Law cannot explain gravity. Gravity is a different fundamental force described by Newton’s Law of Universal Gravitation and later by Einstein’s General Theory of Relativity.
Coulomb's law is a captivating subject, but there's even more to explore! Delve into the enigmatic world of electrostatics and unravel the mysteries behind electric interactions. If you're curious about the mind-blowing aspects of magnetic repulsion, we've got you covered there too.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
