Carbon tetrachloride is a chemical compound with a rich history and diverse applications. Known for its role in fire extinguishers and refrigeration, this compound has also been used in dry cleaning and as a solvent. However, its use has declined due to health and environmental concerns. Carbon tetrachloride is a clear, heavy liquid with a sweet smell, but don't let its pleasant aroma fool you—it's toxic and can cause serious health issues. Understanding its properties, uses, and risks is crucial for anyone dealing with chemicals. Ready to dive into 50 intriguing facts about carbon tetrachloride? Let's get started!
Key Takeaways:
- Carbon tetrachloride, a once widely used chemical, has harmful health and environmental effects. Regulations and safer alternatives are crucial for a safer future.
- Despite its dangers, carbon tetrachloride has some intriguing historical uses and chemical properties. Ongoing research and public awareness are shaping its future.
What is Carbon Tetrachloride?
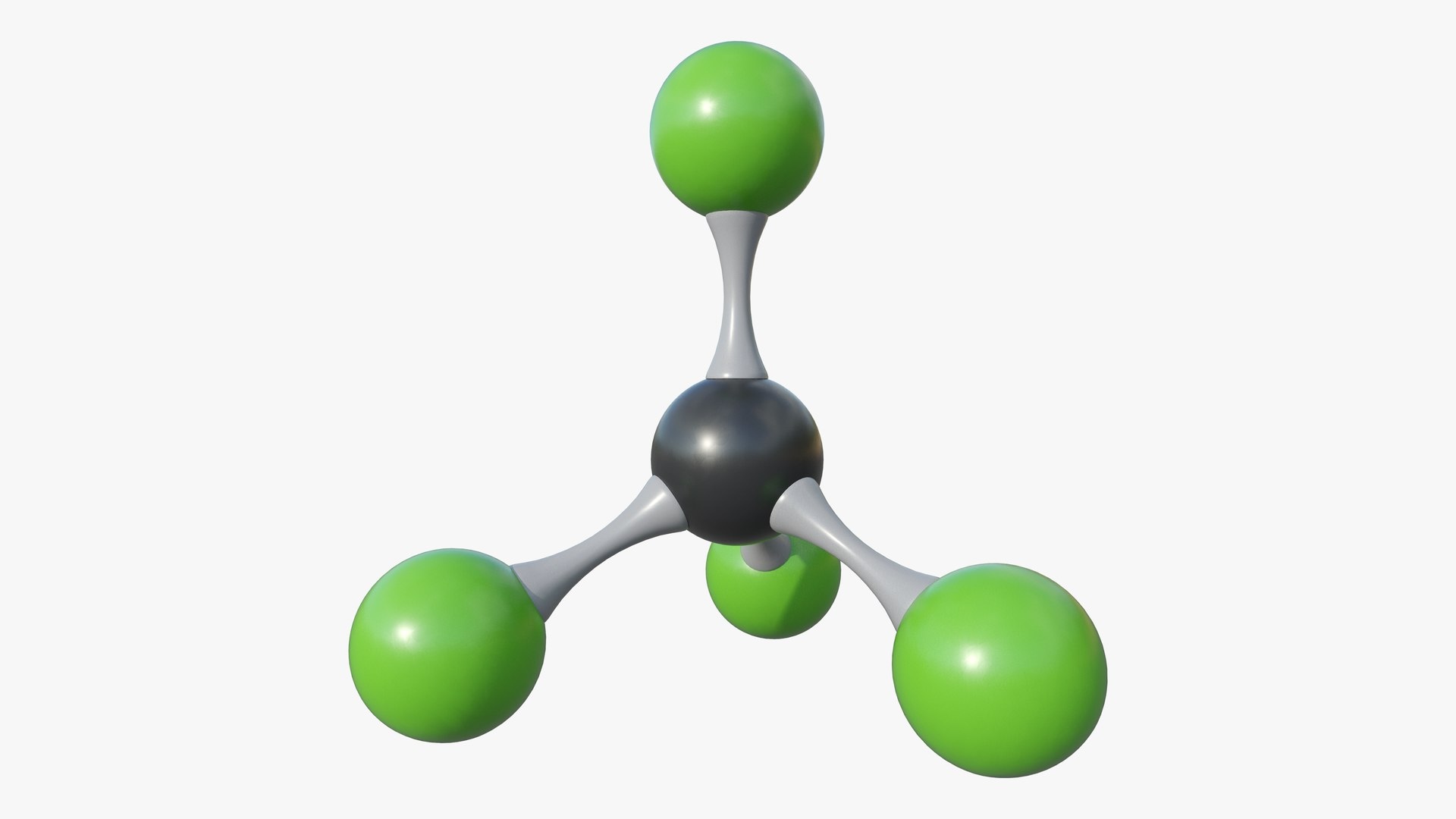
Carbon tetrachloride, also known as tetrachloromethane, is a chemical compound with the formula CCl₄. It has been widely used in various industries but has also raised health and environmental concerns. Here are some intriguing facts about this compound.
-
Carbon tetrachloride is a colorless liquid with a sweet smell, similar to chloroform.
-
It was first synthesized in 1839 by French chemist Henri Victor Regnault.
-
This compound is non-flammable, making it useful in fire extinguishers.
-
It has been used as a refrigerant in the past, although this use has declined due to safety concerns.
-
Carbon tetrachloride is a volatile organic compound (VOC), meaning it easily evaporates into the air.
Uses of Carbon Tetrachloride
Despite its dangers, carbon tetrachloride has had various applications in different fields. Here are some notable uses.
-
It was once a popular solvent for fats, oils, and greases.
-
In the early 20th century, it was commonly used in dry cleaning.
-
The compound has been used in the production of refrigerants like Freon.
-
It served as a pesticide, particularly for killing insects in grain storage.
-
Carbon tetrachloride was an ingredient in some cleaning products and degreasers.
Health Effects of Carbon Tetrachloride
Exposure to carbon tetrachloride can have serious health implications. Here are some facts about its impact on human health.
-
Inhalation of carbon tetrachloride can cause dizziness, headache, and nausea.
-
Prolonged exposure can lead to liver damage and even liver cancer.
-
It can also affect the kidneys, potentially causing kidney failure.
-
Skin contact with carbon tetrachloride can result in irritation and burns.
-
The compound is classified as a Group 2B carcinogen by the International Agency for Research on Cancer (IARC), meaning it is possibly carcinogenic to humans.
Environmental Impact of Carbon Tetrachloride
Carbon tetrachloride has significant effects on the environment. Here are some key points.
-
It contributes to the depletion of the ozone layer.
-
The compound is persistent in the environment, meaning it does not break down easily.
-
It can contaminate soil and groundwater, posing risks to ecosystems.
-
Carbon tetrachloride is toxic to aquatic life, affecting fish and other organisms.
-
Due to its environmental impact, its use has been heavily regulated and reduced over the years.
Historical Context of Carbon Tetrachloride
Understanding the history of carbon tetrachloride helps us see how its use has evolved. Here are some historical facts.
-
During World War II, it was used in fire extinguishers for aircraft.
-
In the 1920s and 1930s, it was marketed as a household cleaning product.
-
The compound was once used in the production of chlorofluorocarbons (CFCs), which were popular in refrigeration and aerosol propellants.
-
By the 1970s, concerns about its health and environmental effects began to surface.
-
In 1987, the Montreal Protocol was signed to phase out substances that deplete the ozone layer, including carbon tetrachloride.
Regulations and Safety Measures
Due to its hazards, various regulations and safety measures have been implemented. Here are some important points.
-
The U.S. Environmental Protection Agency (EPA) has set strict limits on carbon tetrachloride emissions.
-
The Occupational Safety and Health Administration (OSHA) regulates workplace exposure to this compound.
-
Many countries have banned or restricted its use in consumer products.
-
Proper disposal of carbon tetrachloride is crucial to prevent environmental contamination.
-
Personal protective equipment (PPE) is recommended when handling this chemical.
Interesting Chemical Properties
Carbon tetrachloride has some unique chemical properties that make it interesting to study. Here are a few.
-
It has a high density, making it heavier than water.
-
The compound is immiscible with water, meaning it does not mix.
-
It has a boiling point of 76.72°C (170.1°F).
-
Carbon tetrachloride is a good solvent for non-polar substances.
-
It can undergo photolysis, breaking down when exposed to light.
Alternatives to Carbon Tetrachloride
Given its risks, safer alternatives have been developed. Here are some examples.
-
Tetrachloroethylene (PERC) is now more commonly used in dry cleaning.
-
Hydrofluorocarbons (HFCs) have replaced CFCs in refrigeration.
-
Non-toxic solvents like water-based cleaners are used in many industries.
-
Carbon dioxide (CO₂) cleaning is an eco-friendly alternative for some applications.
-
Natural pesticides are preferred over chemical ones for grain storage.
Fun Facts about Carbon Tetrachloride
Despite its dangers, carbon tetrachloride has some fascinating aspects. Here are a few fun facts.
-
It was once used in the production of lava lamps.
-
The compound has been featured in various movies and TV shows as a dangerous chemical.
-
It has a molecular weight of 153.82 g/mol.
-
Carbon tetrachloride has a refractive index of 1.4607.
-
It was used in early experiments to study the properties of gases.
Future of Carbon Tetrachloride
The future of carbon tetrachloride is shaped by ongoing research and regulation. Here are some forward-looking facts.
-
Scientists are studying ways to safely break down and dispose of carbon tetrachloride.
-
Research is ongoing to find even safer alternatives for its various applications.
-
International agreements continue to aim at reducing its environmental impact.
-
Advances in technology may lead to better detection and monitoring of carbon tetrachloride in the environment.
-
Public awareness about the dangers of carbon tetrachloride is increasing, leading to more informed choices and safer practices.
Final Thoughts on Carbon Tetrachloride
Carbon tetrachloride, a chemical with a complex history, has played significant roles in various industries. From its early use as a cleaning agent to its later applications in refrigeration and fire extinguishers, this compound has shown both utility and risk. Its impact on health and the environment led to stricter regulations and a decline in usage. Understanding the dangers and benefits of carbon tetrachloride helps us appreciate the importance of safety and innovation in chemical applications. As we move forward, the lessons learned from carbon tetrachloride's history remind us to balance progress with caution. This knowledge ensures we continue to develop safer, more sustainable solutions for future generations.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
