Salicylaldehyde is a fascinating organic compound with a rich history and diverse applications. But what exactly is salicylaldehyde? In simple terms, it's an aromatic aldehyde derived from salicylic acid. This compound is known for its pleasant almond-like aroma and is used in various industries, from perfumery to pharmaceuticals. Why should you care about salicylaldehyde? Because it's not just a chemical; it's a key player in creating fragrances, flavorings, and even certain medications. Understanding its properties and uses can give you a deeper appreciation for the science behind everyday products. Ready to learn more? Let's dive into 40 intriguing facts about this versatile compound!
Key Takeaways:
- Salicylaldehyde, with its sweet aroma and versatile properties, is used in perfumes, dyes, and even potential cancer research. However, it requires careful handling due to its potential skin and respiratory irritations.
- This compound, found in some plants and essential oils, has a rich history and ongoing research for new drug development and environmental remediation. Its unique properties make it a staple in organic chemistry education.
What is Salicylaldehyde?
Salicylaldehyde is an organic compound with a unique aroma and chemical properties. It's a key ingredient in various industrial applications and scientific research. Let's dive into some fascinating facts about this versatile compound.
Chemical Properties
Understanding the chemical properties of salicylaldehyde helps in grasping its applications and behavior in different environments.
-
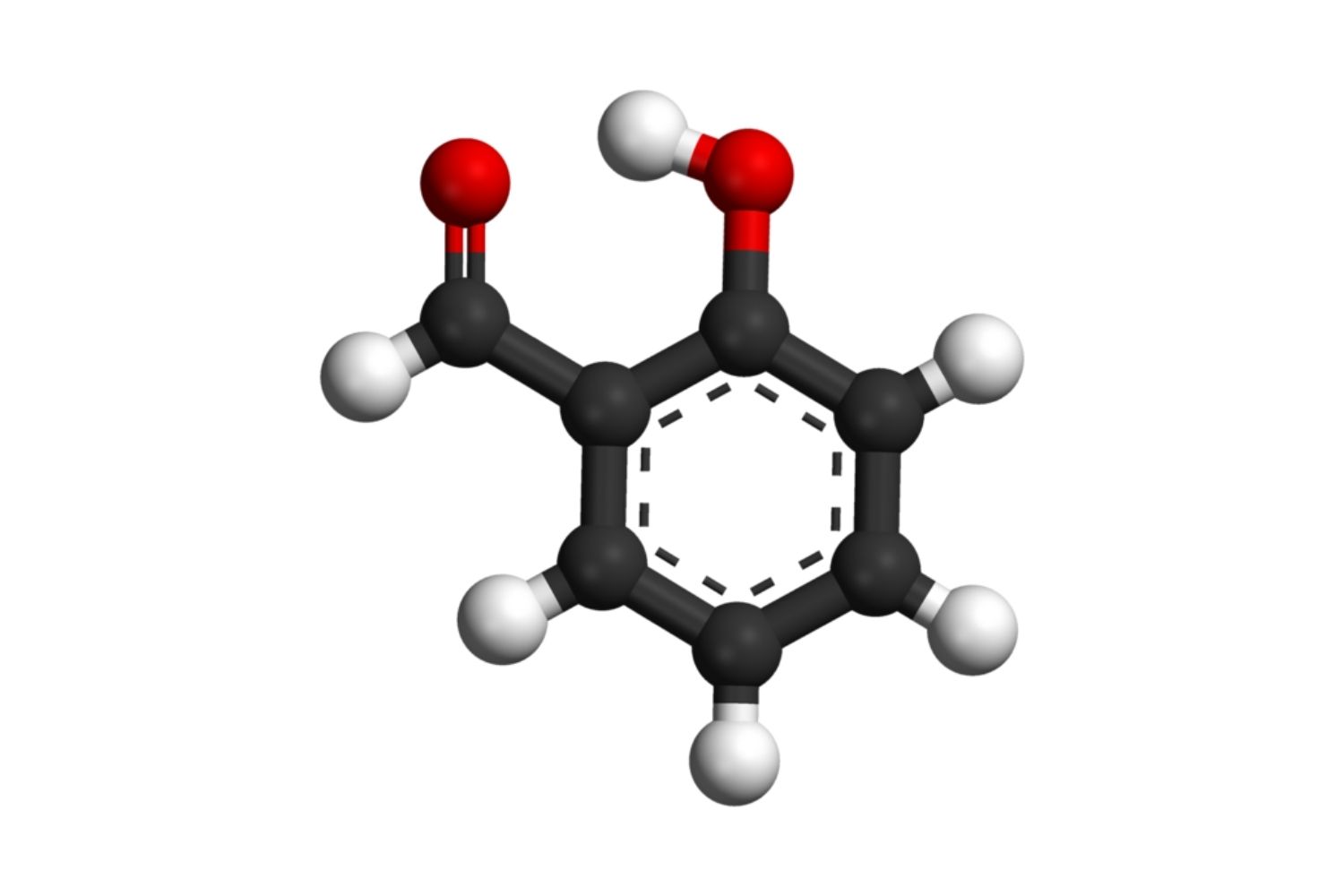
Salicylaldehyde's chemical formula is C7H6O2. This simple structure consists of a benzene ring with an aldehyde and hydroxyl group attached.
-
It has a molecular weight of 122.12 g/mol. This relatively low molecular weight makes it easy to handle in various chemical processes.
-
The compound is slightly soluble in water. This solubility allows it to be used in aqueous solutions for certain reactions.
-
Salicylaldehyde has a melting point of -7°C. This low melting point indicates it remains liquid at room temperature, facilitating its use in liquid form.
-
Its boiling point is 196°C. This high boiling point ensures stability under normal laboratory conditions.
Applications in Industry
Salicylaldehyde's unique properties make it valuable in several industrial applications.
-
Used in the synthesis of coumarin. Coumarin, a fragrant organic chemical, is often derived from salicylaldehyde.
-
Acts as a precursor in pharmaceuticals. Many drugs use salicylaldehyde as a starting material for synthesis.
-
Utilized in the production of dyes. Its chemical structure allows it to be a building block for various dye compounds.
-
Employed in the manufacture of perfumes. The aromatic nature of salicylaldehyde makes it a popular choice in fragrance production.
-
Serves as a chelating agent. In coordination chemistry, it helps in binding metal ions.
Biological Significance
Salicylaldehyde also plays a role in biological systems and research.
-
Found in some plants. Certain plants produce salicylaldehyde as a secondary metabolite.
-
Exhibits antimicrobial properties. It can inhibit the growth of some bacteria and fungi.
-
Used in enzyme studies. Researchers use it to study enzyme interactions and functions.
-
Potential anti-inflammatory effects. Some studies suggest it might help reduce inflammation.
-
Investigated for cancer research. Scientists are exploring its potential in cancer treatment.
Safety and Handling
Knowing how to safely handle salicylaldehyde is crucial for anyone working with this compound.
-
Can cause skin irritation. Direct contact may lead to irritation or allergic reactions.
-
Inhalation may be harmful. Breathing in vapors can cause respiratory issues.
-
Should be stored in a cool, dry place. Proper storage conditions help maintain its stability.
-
Use protective equipment when handling. Gloves and goggles are recommended to prevent exposure.
-
Dispose of according to local regulations. Proper disposal ensures environmental safety.
Historical Context
Salicylaldehyde has an interesting history that has shaped its current uses.
-
First isolated in the 19th century. Early chemists discovered it while studying plant extracts.
-
Named after the willow tree. The name "salicyl" comes from Salix, the Latin word for willow.
-
Used in early medicine. Before modern pharmaceuticals, it was part of traditional remedies.
-
Contributed to the development of aspirin. Its structure helped chemists understand how to synthesize acetylsalicylic acid.
-
Studied extensively in organic chemistry. Its simple yet versatile structure makes it a favorite among chemists.
Environmental Impact
Understanding the environmental impact of salicylaldehyde is essential for sustainable use.
-
Biodegradable under certain conditions. It can break down naturally in the environment.
-
Potential aquatic toxicity. High concentrations may harm aquatic life.
-
Low volatility reduces air pollution. Its low tendency to evaporate minimizes its presence in the atmosphere.
-
Can be absorbed by soil. This absorption affects its mobility and degradation in the environment.
-
Monitored in industrial waste. Regulations ensure that its release into the environment is controlled.
Fun Facts
Let's explore some quirky and lesser-known facts about salicylaldehyde.
-
Has a sweet, almond-like smell. This pleasant aroma makes it popular in perfumery.
-
Used in flavoring agents. Some food products use it to enhance flavor.
-
Appears in some essential oils. Its presence adds to the therapeutic properties of these oils.
-
Can be synthesized from phenol. This common laboratory method showcases its versatility.
-
Part of the benzaldehyde family. It shares similarities with other aromatic aldehydes.
Research and Development
Ongoing research continues to uncover new uses and properties of salicylaldehyde.
-
Explored for new drug development. Scientists are investigating its potential in creating new medications.
-
Studied for its antioxidant properties. Research suggests it may help combat oxidative stress.
-
Used in material science. Its chemical properties make it useful in developing new materials.
-
Investigated for environmental remediation. Potential applications include cleaning up pollutants.
-
Part of ongoing chemical education. Its simple structure makes it a staple in teaching organic chemistry.
Final Thoughts on Salicylaldehyde
Salicylaldehyde, a fascinating compound, plays a crucial role in various fields. From its use in perfumes to its importance in organic synthesis, this chemical has a wide range of applications. Its unique properties make it valuable in creating flavors, fragrances, and even pharmaceuticals. Understanding these facts about salicylaldehyde can help appreciate its significance in everyday products.
Whether you're a chemistry enthusiast or just curious, knowing about salicylaldehyde's versatility adds a layer of appreciation for the science behind many common items. This compound's impact on different industries highlights the importance of chemistry in our daily lives. So next time you encounter a pleasant scent or a useful product, remember the role salicylaldehyde might have played in its creation. Keep exploring and learning about the amazing world of chemicals and their contributions to our world.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
