Cyanogen chloride is a chemical compound that might sound like something out of a sci-fi movie, but it's very real and has some interesting uses and properties. What exactly is cyanogen chloride? It's a colorless, highly toxic gas with a pungent odor, often used in chemical synthesis and as a fumigant. This compound can be dangerous, but it also plays a crucial role in various industrial processes. In this blog post, we'll dive into 40 intriguing facts about cyanogen chloride, shedding light on its chemical structure, applications, safety measures, and much more. Get ready to learn some surprising details about this fascinating yet hazardous substance!
Key Takeaways:
- Cyanogen, a toxic gas with a pungent odor, has diverse uses from rocket propellants to early photography. Its presence in space offers insights into the universe and has even made its way into popular culture.
- Despite its serious nature, cyanogen has some fun and quirky aspects, from its distinct almond-like odor to its appearance in science fiction. It has been referenced in literature, movies, and even video games, making it a fascinating chemical compound with a rich history.
What is Cyanogen?
Cyanogen is a chemical compound with a fascinating history and diverse applications. It is a colorless, toxic gas with a pungent odor, often used in organic synthesis and as a fumigant.
-
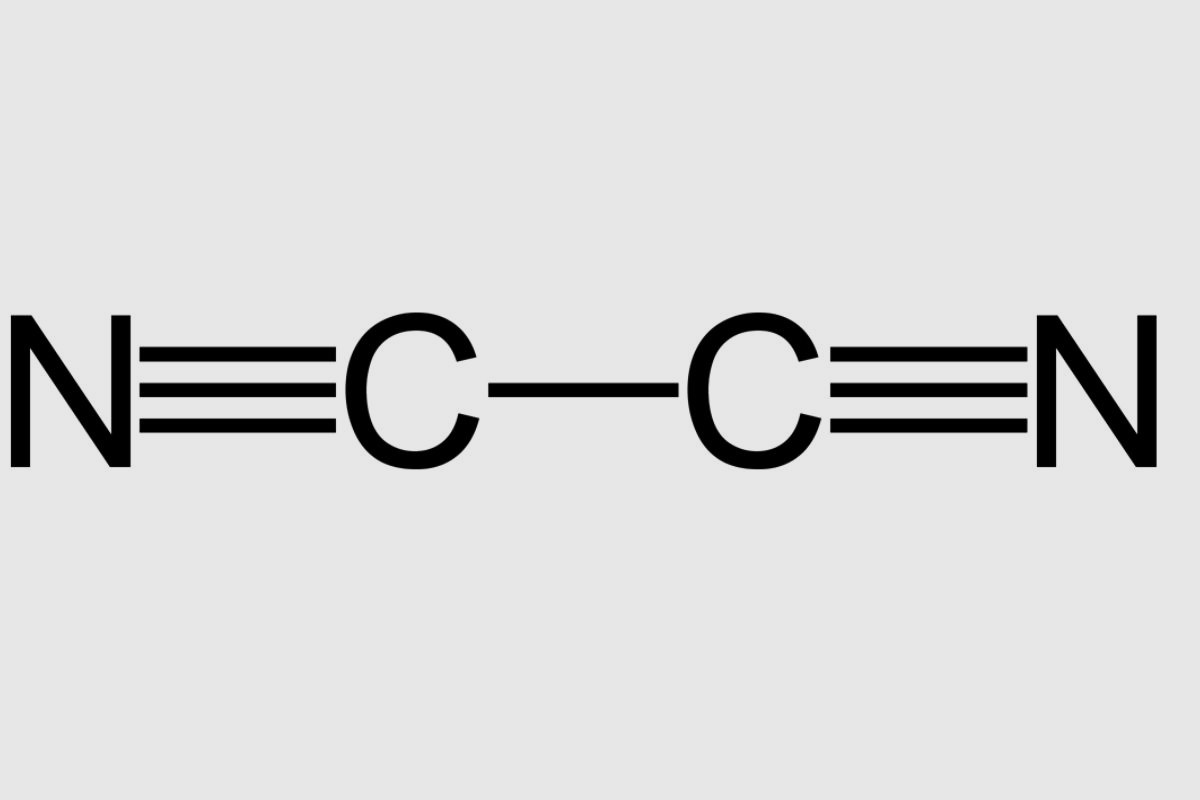
Cyanogen's chemical formula is (CN)₂. This means it consists of two cyanide groups bonded together.
-
It was first discovered in 1815. The French chemist Joseph Louis Gay-Lussac identified cyanogen while studying cyanide compounds.
-
Cyanogen is highly toxic. Exposure can lead to symptoms like headache, dizziness, and even death in high concentrations.
-
It has a boiling point of -21°C (-5.8°F). This makes it a gas at room temperature.
-
Cyanogen is flammable. It can form explosive mixtures with air, making it hazardous to handle.
Uses of Cyanogen
Despite its toxicity, cyanogen has several important uses in various industries. Here are some of the key applications:
-
Used in organic synthesis. Cyanogen is a building block for many organic compounds, including pharmaceuticals and agrochemicals.
-
Serves as a fumigant. It is employed to fumigate ships and silos, killing pests and insects.
-
Used in rocket propellants. Cyanogen can act as a fuel in combination with oxidizers in rocket engines.
-
Involved in the production of cyanogen chloride. This compound is used in chemical synthesis and as a chemical weapon.
-
Used in the mining industry. Cyanogen is employed to extract gold and silver from ores through a process called cyanidation.
Cyanogen in Space
Cyanogen isn't just found on Earth; it also exists in space. Its presence in the cosmos offers intriguing insights into the universe.
-
Detected in comets. Cyanogen has been found in the tails of comets, contributing to their characteristic blue glow.
-
Present in interstellar space. It has been identified in the interstellar medium, the matter that exists between stars.
-
Found in the atmosphere of Titan. Cyanogen is present in the atmosphere of Saturn's moon Titan, indicating complex chemical processes.
-
Detected in the Orion Nebula. This star-forming region contains cyanogen, providing clues about the formation of stars and planets.
-
Used to study the early solar system. The presence of cyanogen in ancient meteorites helps scientists understand the conditions of the early solar system.
Health and Safety Concerns
Given its toxicity, handling cyanogen requires strict safety measures. Here are some important health and safety facts:
-
Exposure limits are very low. Occupational exposure limits are set at 10 parts per million (ppm) to prevent poisoning.
-
Can cause respiratory issues. Inhalation of cyanogen can lead to severe respiratory distress and lung damage.
-
Requires specialized equipment for handling. Gas masks and protective clothing are essential when working with cyanogen.
-
Emergency procedures are critical. In case of exposure, immediate medical attention and decontamination are necessary.
-
Used in chemical warfare. Cyanogen chloride, a related compound, has been used as a chemical weapon due to its toxic effects.
Environmental Impact
Cyanogen's impact on the environment is another important aspect to consider. Here are some key points:
-
Breaks down in the atmosphere. Cyanogen reacts with oxygen and other chemicals, breaking down into less harmful substances.
-
Can contaminate water sources. Accidental releases can lead to water contamination, affecting aquatic life.
-
Used in controlled fumigation. When used as a fumigant, strict controls are in place to minimize environmental impact.
-
Monitored in industrial processes. Industries using cyanogen must adhere to regulations to prevent environmental contamination.
-
Research on biodegradation. Scientists are studying ways to biodegrade cyanogen using microorganisms to reduce its environmental footprint.
Historical Significance
Cyanogen has played a role in various historical events and scientific discoveries. Here are some notable historical facts:
-
Used in early photography. Cyanogen compounds were used in the development of early photographic processes.
-
Involved in the discovery of cyanide. The study of cyanogen led to the identification of cyanide, a highly toxic compound.
-
Part of early rocket experiments. Cyanogen was used in early experiments with rocket propulsion.
-
Studied by famous chemists. Chemists like Gay-Lussac and Justus von Liebig conducted significant research on cyanogen.
-
Linked to the development of synthetic dyes. Cyanogen derivatives were used in the creation of synthetic dyes in the 19th century.
Fun Facts About Cyanogen
Despite its serious nature, cyanogen has some interesting and quirky aspects. Here are a few fun facts:
-
Has a distinct almond-like odor. Some people can detect its smell, similar to bitter almonds.
-
Used in magic tricks. Cyanogen gas can create impressive flames, making it a favorite in some magic performances.
-
Appears in science fiction. Cyanogen has been featured in various science fiction stories and movies as a dangerous chemical.
-
Can form beautiful crystals. When cooled, cyanogen can crystallize into stunning, needle-like structures.
-
Part of the cyanogen bromide test. This test is used in biochemistry to identify certain amino acids in proteins.
Cyanogen in Popular Culture
Cyanogen has made its way into popular culture in various ways. Here are some examples:
-
Referenced in literature. Authors like H.G. Wells have mentioned cyanogen in their works.
-
Appears in movies. Films about chemical warfare and space exploration often include references to cyanogen.
-
Used in video games. Some video games feature cyanogen as a dangerous substance players must avoid.
-
Mentioned in music. Some songs and albums have titles or lyrics referencing cyanogen.
-
Featured in educational programs. Science shows and documentaries often discuss cyanogen's properties and uses.
The Final Word on Cyanogenesis
Cyanogenesis, the process by which certain plants produce cyanide, plays a crucial role in plant defense. This fascinating mechanism helps plants deter herbivores and pests. While cyanide is toxic, many plants have evolved to safely store and release it only when needed.
Understanding cyanogenesis can help us appreciate the complexity of plant biology. It also has practical applications in agriculture and medicine. For instance, knowing which plants produce cyanide can guide farmers in crop selection and pest management. Additionally, researchers are exploring ways to harness cyanogenesis for developing new drugs.
In short, cyanogenesis is a remarkable example of nature's ingenuity. It highlights the delicate balance between survival and adaptation. So next time you see a cassava or an apple seed, remember the hidden chemistry at play. Knowledge of cyanogenesis not only enriches our understanding but also opens doors to innovative solutions.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
